Contents
- 1 Monoclonal Antibodies
- 1.0.1 Uses of Monoclonal Antibodies
- 1.0.2 Monoclonal Antibodies Used for COVID-19
- 1.0.3 List of FDA-approved Monoclonal Antibodies
- 1.0.4 Side Effects of Monoclonal Antibodies
- 1.0.5 Drug Interactions with Monoclonal Antibodies
- 1.0.6 Safety of Monoclonal Antibody Therapy During Pregnancy and Breastfeeding
- 1.0.7 Subscribe to MedicineNet’s Cancer Report Newsletter
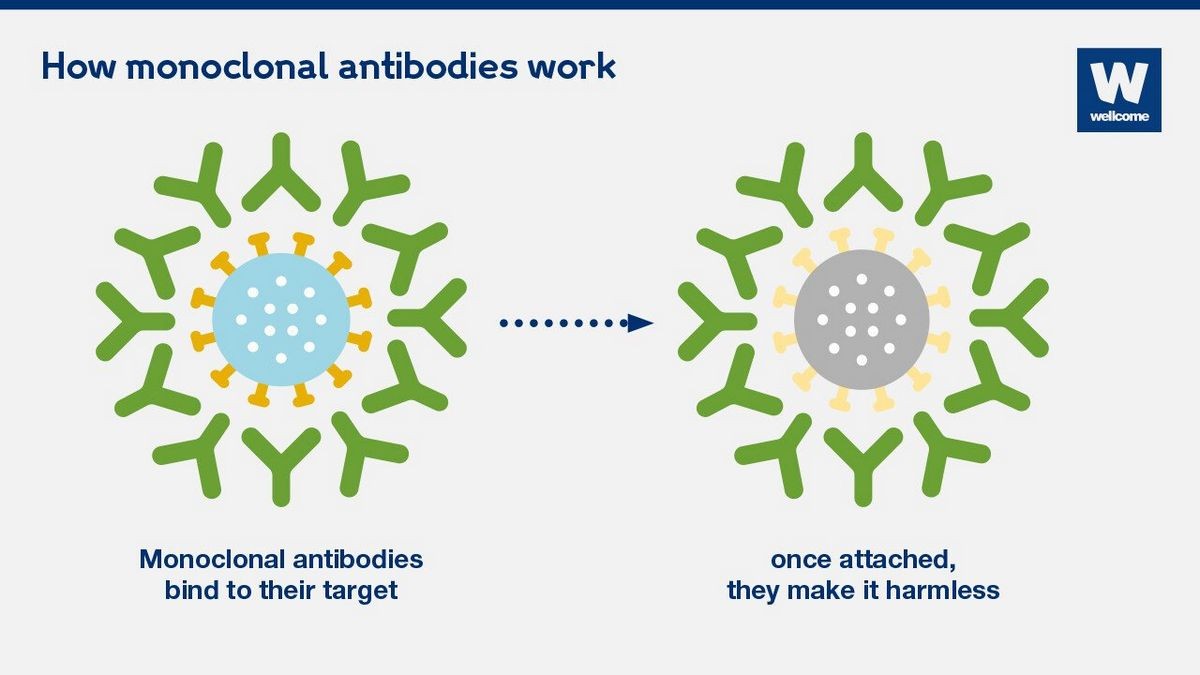
Monoclonal Antibodies
An antibody is a protein produced by the immune system in response to harmful substances called antigens. Antigens can include bacteria, fungi, parasites, viruses, and chemicals that the immune system recognizes as foreign.
Sometimes, the immune system mistakenly produces antibodies against the body’s own tissues, leading to autoimmune conditions like rheumatoid arthritis and multiple sclerosis (MS).
While antibodies are naturally produced by the immune system, scientists can also create them in the lab to mimic the immune system’s action. These man-made antibodies, produced by introducing human genes into mice or other mammals, target specific proteins that attack normal tissues in people with autoimmune disorders. Monoclonal antibodies are synthesized from cloned immune cells and bind to only one type of antigen, while polyclonal antibodies are synthesized from different immune cells and bind to multiple antigens.
Monoclonal antibody therapy has been authorized for emergency use in treating COVID-19.
Uses of Monoclonal Antibodies
Monoclonal antibodies are used in immunotherapy to target specific antigens in the body. They are utilized in various conditions, including:
- Cancer
- Rheumatoid arthritis
- Multiple sclerosis
- Cardiovascular disease
- Systemic lupus erythematosus
- Crohn’s disease
- Ulcerative colitis
- Psoriasis
- COVID-19
- Transplant rejection, and more
In these conditions, monoclonal antibodies interfere with the action of specific chemicals or receptors involved in the development of the treated condition. For example, a monoclonal antibody used for cancer treatment may block a receptor that cancer cells use to evade the immune system. Blocking this receptor allows the immune system to recognize and destroy cancer cells.
Monoclonal antibodies are administered through injections and are available as lyophilized powder for reconstitution or solution for injection.
Monoclonal Antibodies Used for COVID-19
The FDA has authorized two monoclonal antibody medications for emergency use in COVID-19 treatment: bebtelovimab and tixagevimab/cilgavimab. Bebtelovimab is used for outpatients only and not for hospitalized patients with severe symptoms. Tixagevimab/cilgavimab is used as a preventive measure before exposure to COVID-19 and does not treat symptoms or prevent illness after exposure. It should not replace COVID vaccination.
Some monoclonal antibody drugs have been paused in the United States due to their lack of effectiveness against the Omicron variant of COVID-19. These drugs include bamlanivimab plus etesevimab, casirivimab plus imdevimab, and sotrovimab. Ongoing studies continue to explore the usefulness and safety of COVID-19 antibody treatments.
List of FDA-approved Monoclonal Antibodies
Here are some examples of FDA-approved monoclonal antibody drugs:
- abciximab (Reopro)
- adalimumab (Humira, Amjevita)
- alefacept (Amevive)
- alemtuzumab (Campath)
- basiliximab (Simulect)
- belimumab (Benlysta)
- bezlotoxumab (Zinplava)
- canakinumab (Ilaris)
- certolizumab pegol (Cimzia)
- cetuximab (Erbitux)
- daclizumab (Zenapax, Zinbryta)
- denosumab (Prolia, Xgeva)
- efalizumab (Raptiva)
- golimumab (Simponi, Simponi Aria)
- inflectra (Remicade)
- ipilimumab (Yervoy)
- ixekizumab (Taltz)
- natalizumab (Tysabri)
- nivolumab (Opdivo)
- olaratumab (Lartruvo)
- omalizumab (Xolair)
- palivizumab (Synagis)
- panitumumab (Vectibix)
- pembrolizumab (Keytruda)
- rituximab (Rituxan)
- tocilizumab (Actemra)
- trastuzumab (Herceptin)
- secukinumab (Cosentyx)
- ustekinumab (Stelara)
Each monoclonal antibody listed above has a unique role in treating specific diseases.
What Is Polycythemia Vera?
Side Effects of Monoclonal Antibodies
The following side effects are common but can vary among different monoclonal antibodies:
- Shortness of breath
- Peripheral edema
- Headache
- Fever
- Muscle aches and pain
- Decreased appetite
- Increased triglyceride levels
- Insomnia
- Abdominal pain
- Back pain
- Dizziness
Serious side effects of monoclonal antibodies can include:
- Low blood pressure
- Anaphylaxis
- Serious infections
- Cancer
- Serum sickness
- Autoimmune thyroiditis
- Arterial and venous blood clots
- Congestive heart failure
- Bleeding
- Interstitial lung disease
- Hepatitis
- Generation of antibodies
- Enterocolitis
- Gastrointestinal perforation
- Mucositis
- Stomatitis
- Anemia
- Reduced white blood cell counts
- Hypothyroidism
Drug Interactions with Monoclonal Antibodies
- Serious infections are more likely when monoclonal antibodies are combined with immune-suppressing drugs like steroids.
- Methotrexate reduces the absorption of adalimumab by 29%-49%, but adjustments to the dose of adalimumab are not necessary when taken with methotrexate.
- Monoclonal antibodies may interfere with the effectiveness of vaccines. Live vaccines, including attenuated vaccines, should not be used while receiving monoclonal antibodies. Patients should complete all recommended immunizations before starting monoclonal antibody treatment.
Safety of Monoclonal Antibody Therapy During Pregnancy and Breastfeeding
- Monoclonal antibodies have not been adequately studied in pregnant women or breastfeeding women. Some monoclonal antibodies may be harmful to the fetus based on their mechanism of action and animal studies.
- The presence of monoclonal antibodies in breast milk is unknown. Breastfeeding mothers should consider the risks and benefits and decide whether to continue or discontinue monoclonal antibody treatment due to the potential adverse effects on infants.
Subscribe to MedicineNet’s Cancer Report Newsletter
By clicking "Submit," I agree to the MedicineNet Terms and Conditions and Privacy Policy. I also agree to receive emails from MedicineNet and understand that I may opt out of MedicineNet subscriptions at any time.