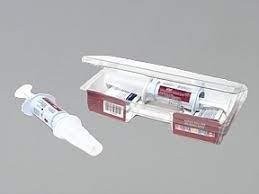
diazepam – rectal, Diastat
Medication Uses: This medication is used to treat episodes of increased seizures (e.g., acute repetitive seizures, breakthrough seizures) in people who are already taking medications to control their seizures. This product is only recommended for short-term treatment of seizure attacks. It is not for ongoing daily use to prevent seizures. Uncontrolled seizures can turn into serious (possibly fatal) seizures that do not stop (status epilepticus). Diazepam belongs to a class of medications called benzodiazepines which produce a calming effect on the brain and nerves (central nervous system). It is thought to work by increasing the effect of a certain natural chemical (GABA) in the brain.
HOW TO USE: Read the Patient/Caregiver Information Leaflet provided by your pharmacist before you use this product and each time you get a refill. If you have questions, consult your doctor or pharmacist. This drug is given rectally by a caregiver trained to recognize the symptoms of your seizures and to correctly give the product. You and your caregivers must follow all instructions from your doctor and pharmacist exactly. Review all the instructions on how to give this medication in the product package. If you have any questions or feel unsure about using this medication, call the doctor or pharmacist before using this product. Get emergency help if the person is having a seizure and you do not feel comfortable using this product. Before using, check the syringe for the correct dose. Your pharmacist should set the correct dose and lock the syringe in the "ready" position before giving you the product. Before leaving the pharmacy, look at each syringe. The dose should be in the display window on the side. You should see a green band with the word "ready" at the bottom of the syringe barrel. Look to make sure you have the correct syringe tip (e.g., smaller tip for a child) and that there are no cracks around the syringe tip. Return the product to the pharmacist if there is a problem or if you have any questions. Cracks can cause the medication to leak out and not provide the correct amount of medication. If you see a crack, use a different syringe. Cracks can appear over time, so keep checking your syringes to make sure you have good ones ready to use. Also check the expiration date on the package, and refill your prescription before the medication expires. The dosage is based on age, weight, medical condition, and response to therapy. Be sure you understand when this medication should be used, how to use it, and how to check for side effects/seizure control. In some cases, a second dose may be prescribed and given 4 to 12 hours after the first dose. Usually, this medication should not be used to treat more than 5 episodes per month and no more than one episode every 5 days. If seizures continue after using this product as prescribed (e.g., no change 15 minutes after dose is given), or if there is a change in the person’s breathing, behavior, or condition that alarms you, get emergency help right away. This medication should not be used regularly. This medication may cause withdrawal reactions, especially if it has been used regularly for a long time (more than a few weeks) or in high doses. In such cases, withdrawal symptoms (such as increased seizures) may occur if you suddenly stop using this medication. To prevent withdrawal reactions, your doctor may reduce your dose gradually. Consult your doctor or pharmacist for more details, and report any withdrawal reactions immediately. Though it is very unlikely to occur, this medication can also result in abnormal drug-seeking behavior (addiction/habit forming). Do not increase your dose, take it more frequently, or use it for a longer time than prescribed. Dispose of this medication properly. Follow the directions in the Patient Information Leaflet. Do not reuse the syringe. Do not stop taking your regular seizure control medications when you are given this drug.
SIDE EFFECTS: Drowsiness, dizziness, diarrhea, and unsteadiness may occur. If these persist or worsen, notify your doctor promptly. Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects. This medication stays in the body for a long time. Be sure to watch for reactions for at least 4 hours after giving the medication. Seek immediate medical attention if any of these unlikely but very serious side effects occur: slow/shallow/difficult breathing, mental/mood changes (e.g., anxiety, restlessness, hallucinations, sleep problems), slurred speech, trouble walking. A serious allergic reaction to this drug is unlikely, but seek immediate medical attention if it occurs. Symptoms of a serious allergic reaction include: rash, itching/swelling (especially of the face/tongue/throat), dizziness, trouble breathing. This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist. PRECAUTIONS: Before using this product, tell your doctor or pharmacist if you are allergic to it; or to other benzodiazepines (e.g., oxazepam, temazepam); or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details. This medication should not be used if you have certain medical conditions. Before using this medicine, consult your doctor or pharmacist if you have: a certain eye problem (narrow-angle glaucoma), a certain muscle disease (myasthenia gravis), breathing trouble during sleep (sleep apnea). Before using this medication, tell your doctor or pharmacist your medical history, especially of: breathing problems (e.g., asthma, pneumonia), brain problems that could affect breathing (e.g., decreased consciousness, head injury), a certain eye problem (wide-angle glaucoma), kidney disease, liver disease, history of drug/alcohol abuse. This drug may make you dizzy or drowsy. Do not drive, use machinery, ride a bicycle, or do any activity that requires alertness until you are sure you can perform such activities safely. Avoid alcoholic beverages. To minimize dizziness and lightheadedness, get up slowly when rising from a sitting or lying position. Older adults may be more sensitive to the side effects of this drug, especially drowsiness and loss of coordination. These side effects can increase the risk of falling. This medication is not recommended for use during pregnancy. It may harm an unborn baby. However, since untreated seizures are a serious condition that can harm both a pregnant woman and her unborn baby, do not stop taking this medication unless directed by your doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately talk to your doctor about the benefits and risks of using this medication during pregnancy. This drug may pass into breast milk. Because of the possible harm to the infant, breastfeeding while using this drug is not recommended. Consult your doctor before breastfeeding.
QUESTION
DRUG INTERACTIONS: Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor’s approval. Some products that may interact with this drug include: cimetidine, clozapine, fluvoxamine, sodium oxybate. Tell your doctor or pharmacist if you also take drugs that cause drowsiness such as: antidepressants (e.g., amitriptyline, nefazodone), certain antihistamines (e.g., diphenhydramine), anti-seizure drugs (e.g., carbamazepine, phenobarbital, valproate), medicine for sleep or anxiety (e.g., alprazolam, kava, zolpidem), muscle relaxants, narcotic pain relievers (e.g., codeine), psychiatric medicines (e.g., chlorpromazine, risperidone). This medication contains a small amount of alcohol. Tell your doctor if you are taking drugs such as disulfiram or metronidazole that can cause an unpleasant reaction when combined with alcohol. Avoid alcohol when using this medication because it may increase side effects such as difficulty breathing and drowsiness. Check the labels on all your medicines (e.g., cough-and-cold products) because they may contain drowsiness-causing ingredients. Ask your pharmacist about the safe use of those products. OVERDOSE: This medicine may be harmful if swallowed. If swallowing or overdose is suspected, contact a poison control center or emergency room immediately. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: confusion, slow reflexes, clumsiness, deep sleep, and loss of consciousness. NOTES: Do not share this medication with others. It is against the law. MISSED DOSE: Not applicable. STORAGE: Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets. See also How to Use section. Properly discard this product when it is expired or no longer needed. Read the Patient/Caregiver Information Leaflet for details. To discard this medication, the FDA recommends flushing down the toilet or pouring into a drain. However, consult your pharmacist or local waste disposal company. MEDICAL ALERT: Your condition can cause complications in a medical emergency. For information about enrolling in MedicAlert, call 1-888-633-4298 (US) or 1-800-668-1507 (Canada). Information last revised February 2014. Copyright(c) 2014 First Databank, Inc.
Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.


