Contents
bivalirudin
Bivalirudin is an anticoagulant medication used to prevent blood clotting in patients undergoing percutaneous coronary intervention (PCI). PCI is a nonsurgical procedure performed using a thin flexible tube (catheter) to place a stent in coronary arteries that have narrowed due to plaque formation (atherosclerosis). Bivalirudin is administered intravenously during the PCI procedure to prevent blood coagulation, and in some instances for a short period post procedure, for instance, in patients who have had a heart attack.
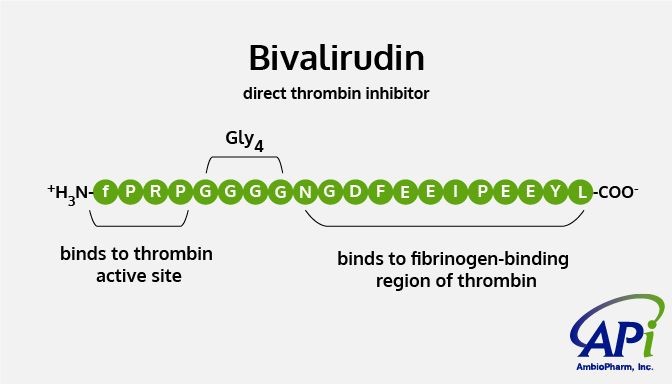
Bivalirudin is a structurally similar analog of hirudin, a peptide naturally produced by the salivary glands of blood-sucking leeches to keep the blood flowing when they bite a human. Bivalirudin is used to prevent blood clotting and obstructing blood flow during PCI procedure with the catheter and the stent placement. Bivalirudin prevents blood clotting by inhibiting thrombin, an enzyme in blood that is involved in the blood-clotting process.
Prothrombin and fibrinogen are inactive forms of special proteins in blood that enable clot formation. During the normal clotting process (coagulation cascade), prothrombin gets converted to thrombin, its active form, which induces platelets in the blood to aggregate and form a plug at the tear in the blood vessel. Thrombin also converts fibrinogen into fibrin, the tough insoluble protein that provides scaffolding and stability to the blood clot. Bivalirudin inhibits thrombin and prevents both these coagulation processes.
Heparin is the commonly used anticoagulant during PCI procedures. Bivalirudin is used in patients in whom heparin cannot be used because they have developed adverse reactions to heparin. In such patients, use of heparin can cause a drop in platelets. Some patients may have developed antibodies to heparin, which can cause a highly coagulable state of blood and life-threatening complications. The FDA-approved uses of bivalirudin are:
- As an anticoagulant in patients undergoing percutaneous coronary intervention (PCI), including patients with:
- Heparin-induced thrombocytopenia and
- Heparin-induced thrombocytopenia and thrombosis syndrome
Warnings
- Do not use bivalirudin in patients with hypersensitivity to any component of the formulation.
- Do not use bivalirudin in patients with significant active bleeding.
- Bivalirudin should be administered intravenously. Do not administer intramuscularly.
- Bivalirudin increases the risk of bleeding, and its effects last for an hour after discontinuation of the drug. The risk of bleeding is higher in elderly patients.
- Monitor patients for signs and symptoms of bleeding during the administration of bivalirudin. Monitor patients with diseases associated with bleeding more frequently.
- Unexplained decrease in blood pressure or hematocrit could signify bleeding. Evaluate immediately and discontinue bivalirudin, if required.
- Patients who develop acute stent thrombosis will likely require a target vessel revascularization procedure.
- Monitor patients for at least 24 hours in a facility where such complications can be handled.
QUESTION
What are the side effects of bivalirudin?
Common side effects of bivalirudin include:
- Minor bleeding events
- Major bleeding events including:
- Intracranial hemorrhage
- Retroperitoneal hemorrhage
Call your doctor immediately if you experience any of the following symptoms or serious side effects while using this drug:
- Serious heart symptoms include fast or pounding heartbeats, fluttering in your chest, shortness of breath, and sudden dizziness;
- Severe headache, confusion, slurred speech, severe weakness, vomiting, loss of coordination, feeling unsteady;
- Severe nervous system reaction with very stiff muscles, high fever, sweating, confusion, fast or uneven heartbeats, tremors, and feeling like you might pass out; or
- Serious eye symptoms include blurred vision, tunnel vision, eye pain or swelling, or seeing halos around lights.
This is not a complete list of all side effects or adverse reactions that may occur from the use of this drug. Call your doctor for medical advice about serious side effects or adverse reactions. You may also report side effects or health problems to the FDA at 1-800-FDA-1088.
What are the dosages of bivalirudin?
Injection, powder for reconstitution
Injection, ready-to-use solution
- 5 mg/mL (250 mg/50 mL; 500 mg/100 mL)
Adult:
Percutaneous Coronary Intervention
- Use as anticoagulant in heparin-induced thrombocytopenia (HIT) and heparin-induced thrombocytopenia and thrombosis (HITT) syndrome
- Has only been studied in patients receiving concomitant aspirin
- 0.75 mg/kg intravenous (IV) bolus, and then IMMEDIATELY 1.75 mg/kg/hour IV infusion for duration of procedure
- Obtain activated clotting time (ACT) 5 min after administering the bolus dose; an additional IV bolus of 0.3 mg/kg should be given if needed
- Consider extended duration of infusion following PCI at 1.75 mg/kg/hour for up to 4 hours post procedure in patients with ST segment elevation MI (STEMI)
Dosage Modifications
- Bolus Dose
- No reduction required for any degree of renal impairment
- Moderate (creatinine clearance [CrCl] 30-59 mL/minute): 1.75 mg/kg/hour
- Severe (CrCl less than 30 mL/minute): 1 mg/kg/hour
- Hemodialysis: 0.25 mg/kg/hour
- No dosage adjustment required
Dosing Considerations
- Safety and effectiveness have not been established in patients with acute coronary syndromes who are not undergoing percutaneous transluminal coronary angioplasty (PTCA) or PCI
Heparin-induced Thrombocytopenia (Off-label)
- Initial: 0.15-0.2 mg/kg/hour IV; adjust to activated partial thromboplastin time (aPTT) 1.5-2.5 times baseline value
Pediatric:
- Safety and efficacy not established
Overdose
- There have been bivalirudin overdose cases of up to 10 times the recommended dosage in clinical trials and post-marketing reports, primarily due to failure of adjusting doses in patients with impaired kidney function.
- Bivalirudin overdose can cause hemorrhage that can result in death.
- If bivalirudin overdose is suspected, discontinue the drug immediately and closely monitor patients for signs of bleeding. Bivalirudin has no known antidote, but can be hemodialyzed.
What drugs interact with bivalirudin?
Inform your doctor of all medications you are currently taking, who can advise you on any possible drug interactions. Never begin taking, suddenly discontinue, or change the dosage of any medication without your doctor’s recommendation.
- Severe interactions of bivalirudin include:
- defibrotide
- mifepristone
- prothrombin complex concentrate, human
The drug interactions listed above are not all of the possible interactions or adverse effects. For more information on drug interactions, visit the RxList Drug Interaction Checker.
It is important to always tell your doctor, pharmacist, or health care provider of all prescription and over-the-counter medications you use, as well as the dosage for each, and keep a list of the information. Check with your doctor or health care provider if you have any questions about the medication.
By clicking Submit, I agree to the MedicineNet’s Terms & Conditions & Privacy Policy and understand that I may opt out of MedicineNet’s subscriptions at any time.
Pregnancy and breastfeeding
- Animal reproductive studies on bivalirudin use during pregnancy do not reveal evidence of fetal harm.
- There are no data on the use of bivalirudin in pregnant women to identify a drug-associated risk for adverse developmental outcomes.
- There are no studies on the use of bivalirudin during labor and delivery because of the potential complications of drug-induced hemorrhage during delivery.
- There is no information on the presence of bivalirudin in breastmilk, or its effects on milk production or the breastfed infant.
- Decision to breastfeed during treatment with bivalirudin should be based on the mother’s clinical need for the drug, the developmental and health benefits of breastfeeding, and potential risks to the breastfed infant from exposure to the drug or from the mother’s underlying condition.
What else should I know about bivalirudin?
- Notify your physician immediately if you notice any signs and symptoms of unusual bleeding or bruising after administration of bivalirudin.


