Contents
Side Effects of Vyvgart (efgartigimod alfa-fcab)
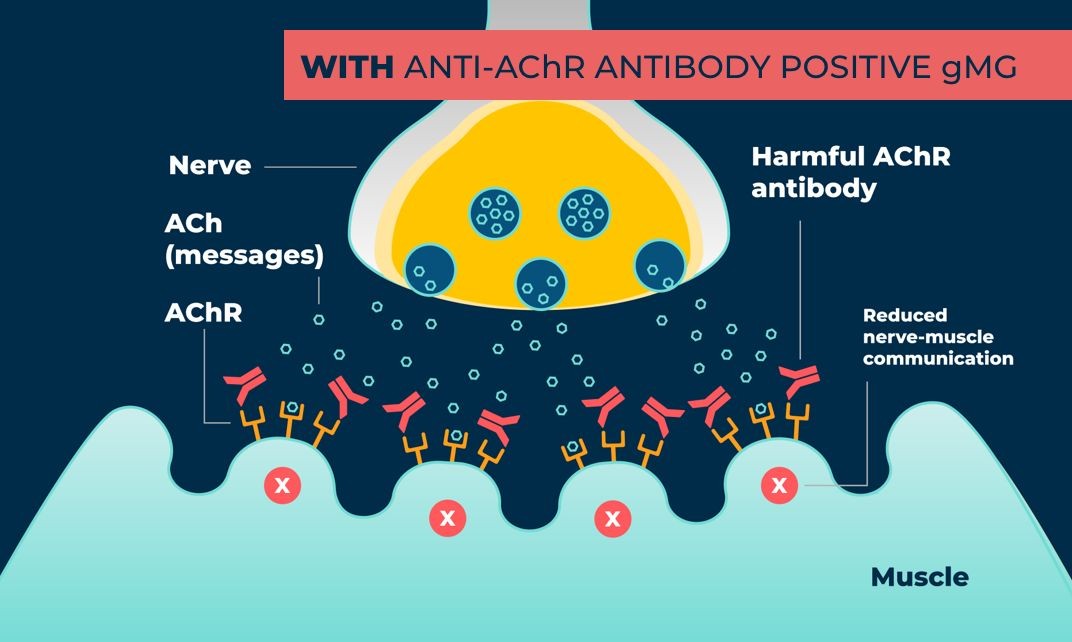
Vyvgart (efgartigimod alfa-fcab) injection for intravenous use is a neonatal Fc receptor blocker indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.
Common side effects of Vyvgart include respiratory tract infections, headache, urinary tract infection (UTI), numbness and tingling, and muscle pain.
Serious side effects of Vyvgart include increased risk of infections and hypersensitivity reactions including rash, skin swelling (angioedema), and shortness of breath.
Drug interactions of Vyvgart include medications that bind to the human neonatal Fc receptor (FcRn) (e.g., immunoglobulin products, monoclonal antibodies, or antibody derivatives containing the human Fc domain of the IgG subclass) because the combination may lower systemic exposures and reduce the effectiveness of such medications.
No available data on the use of Vyvgart during pregnancy. Monoclonal antibodies are increasingly transported across the placenta as pregnancy progresses, with the largest amount transferred during the third semester.
Therefore, efgartigimod alfa-fcab may be transmitted from the mother to the developing fetus. As Vyvgart is expected to reduce maternal IgG antibody levels, reduction in passive protection to the newborn is anticipated. Risk and benefits should be considered prior to administering live or live-attenuated vaccines to infants exposed to Vyvgart in utero.
No information regarding the presence of efgartigimod alfa-fcab in human milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Vyvgart and any potential adverse effects on the breastfed infant from Vyvgart or from the underlying maternal condition.
What are the side effects of Vyvgart?
What are the serious side effects of Vyvgart?
Vyvgart may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- severe dizziness,
- fever, and
- sore throat
Get medical help right away, if you have any of the symptoms listed above.
What are the common side effects of Vyvgart?
The most common side effects of Vyvgart include:
- cough,
- sneezing,
- stuffy or runny nose,
- sore throat,
- shortness of breath,
- wheezing,
- fever,
- headache,
- migraine and procedural headache,
- pain or burning when you urinate,
- numbness and tingling,
- loss of sensation in the mouth,
- decreased sense of touch or sensation,
- increased sensitivity to stimulation, and
- muscle pain or soreness
When to call the doctor
- Tell the doctor if you have any side effect that bothers you or that does not go away.
- These are not all the possible side effects of Vyvgart. For more information, ask your doctor or pharmacist.
- Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Is Vyvgart addictive?
No information provided
What drugs interact with Vyvgart?
Effect Of Vyvgart On Other Drugs
- Concomitant use of Vyvgart with medications that bind to the human neonatal Fc receptor (FcRn) (e.g., immunoglobulin products, monoclonal antibodies, or antibody derivates containing the human Fc domain of the IgG subclass) may lower systemic exposures and reduce effectiveness of such medications.
- Closely monitor for reduced effectiveness of medications that bind to the human neonatal Fc receptor.
- When concomitant long-term use of such medications is essential for patient care, consider discontinuing Vyvgart and using alternative therapies.
Vyvgart side effects list for healthcare professionals
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infections
- Hypersensitivity Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical studies, the safety of Vyvgart has been evaluated in 246 patients who received at least one dose of Vyvgart, including 57 patients exposed to at least 7 treatment cycles and 8 patients exposed to at least 10 treatment cycles.
In a placebo-controlled study (Study 1) in patients with gMG, 84 patients received Vyvgart 10 mg/kg. Of these 84 patients, approximately 75% were female, 82% were White, 11% were Asian, and 8% were of Hispanic or Latino ethnicity. The mean age at study entry was 46 years (range 19 to 78).
The minimum time between treatment cycles, specified by study protocol, was 50 days. On average, Vyvgart-treated patients received 2 cycles in Study 1. The mean and median times to the second treatment cycle were 94 days and 72 days from the initial infusion of the first treatment cycle, respectively, for Vyvgart-treated patients.
Adverse reactions reported in at least 5% of patients treated with Vyvgart and more frequently than placebo are summarized in Table 1. The most common adverse reactions (reported in at least 10% of Vyvgart-treated patients) were respiratory tract infection, headache, and urinary tract infection.
Table 1: Adverse Reactions in ≥ 5% of Patients Treated with Vyvgart and More Frequently than in Placebo-Treated Patients in Study 1 (Safety Population)
| Adverse reaction | Vyvgart (N=84) % |
Placebo (N=83) % |
| Respiratory tract infection | 33 | 29 |
| Headache 1 | 32 | 29 |
| Urinary tract infection | 10 | 5 |
| Paraesthesia 2 | 7 | 5 |
| Myalgia | 6 | 1 |
| 1 Headache includes migraine and procedural headache. 2 Paraesthesia includes oral hypoesthesia, hypoesthesia, and hyperesthesia. |
||
Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Vyvgart in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
In up to 26 weeks of treatment in Study 1, 20% (17/83) of patients developed antibodies to Vyvgart. Seven percent (6/83) of patients developed neutralizing antibodies.
Because few patients tested positive for anti-efgartigimod alfa-fcab antibodies and neutralizing antibodies, the available data are too limited to make definitive conclusions regarding immunogenicity and the effect on pharmacokinetics, safety, or efficacy of Vyvgart.
Summary
Vyvgart (efgartigimod alfa-fcab) injection for intravenous use is a neonatal Fc receptor blocker indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive. Common side effects of Vyvgart include respiratory tract infections, headache, urinary tract infection (UTI), numbness and tingling, and muscle pain. Serious side effects of Vyvgart include increased risk of infections and hypersensitivity reactions including rash, skin swelling (angioedema), and shortness of breath.