Contents
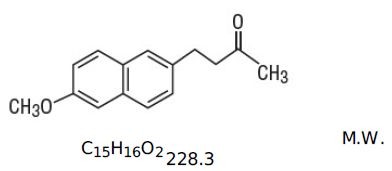
Side Effects of Relafen (nabumetone)
NSAIDs are used to manage mild to moderate pain, fever, and inflammation. They work by reducing prostaglandin levels, chemicals responsible for pain, fever, and inflammation. NSAIDs block the enzyme that produces prostaglandins, resulting in lower concentrations.
Inflammation, pain, and fever are reduced. The brand name drug Relafen is no longer available in the U.S.
Common side effects of Relafen include
Serious side effects of Relafen include
- serious gastrointestinal bleeding (signs may include black, tarry stools, weakness, dizziness upon standing),
- liver toxicity,
- kidney impairment,
- increased bleeding after an injury,
- rare but severe allergic reactions (particularly in patients with a history of exacerbation of asthma, hives, or other allergic reactions to aspirin or other NSAIDs),
- fluid retention (edema),
- blood clots,
- heart attacks,
- high blood pressure (hypertension), and
- heart failure
Drug interactions of Relafen include ACE inhibitors because Relafen may diminish the antihypertensive effect of ACE inhibitors.
- The concomitant administration of Relafen and aspirin is not generally recommended because of the potential of increased adverse effects.
- Relafen can reduce the natriuretic effect of furosemide and thiazides in some patients.
- NSAIDs have produced an elevation of plasma lithium levels and a reduction in lithium clearance via the kidneys.
- NSAIDs could enhance the toxicity of methotrexate.
- Users of both warfarin and NSAIDs together have a risk of serious gastrointestinal bleeding higher than users of either drug alone.
In late pregnancy, as with other NSAIDs, Relafen should be avoided because it may cause premature closure of the ductus arteriosus.
It is unknown if Relafen is excreted in breast milk. Consult your doctor before breastfeeding.
What are the important side effects of Relafen (nabumetone)?
Most patients benefit from nabumetone and other NSAIDs with few side effects. However, serious side effects can occur, and generally tend to be dose-related. Therefore, it is advisable to use the lowest effective dose to minimize side effects. The most common side effects of nabumetone involve the gastrointestinal system:
- abdominal pain,
- cramping,
- nausea,
- gastritis,
- serious gastrointestinal bleeding, and
- liver toxicity.
Sometimes ulceration and bleeding can occur without abdominal pain. Black, tarry stools, weakness, and dizziness upon standing may be the only signs of internal bleeding. Some studies have shown that nabumetone may have a lower risk of gastrointestinal side effects than other NSAIDs.
Other important side effects caused by nabumetone include:
- rash,
- kidney impairment, and
- ringing in the ears.
NSAIDs reduce the ability of blood to clot and therefore increase bleeding after an injury. Nabumetone should be avoided by patients with a history of exacerbation of asthma, hives, or other allergic reactions to aspirin or other NSAIDs. Rare but severe allergic reactions have been reported in such individuals. Fluid retention (edema), blood clots, heart attacks, hypertension, and heart failure have also been associated with the use of NSAIDs.
Relafen (nabumetone) side effects list for healthcare professionals
Adverse reaction information was derived from blinded-controlled and open-labeled clinical trials and from worldwide marketing experience. In the description below, rates of the more common events (greater than 1%) and many of the less common events (less than 1%) represent results of US clinical studies.
Of the 1,677 patients who received Relafen (nabumetone) during US clinical trials, 1,524 were treated for at least 1 month, 1,327 for at least 3 months, 929 for at least a year, and 750 for at least 2 years. More than 300 patients have been treated for 5 years or longer.
The most frequently reported adverse reactions were related to the gastrointestinal tract and included diarrhea, dyspepsia, and abdominal pain.
Incidence ≥ 1%-Probably Causally Related
Gastrointestinal: Diarrhea (14%), dyspepsia (13%), abdominal pain (12%), constipation*, flatulence*, nausea*, positive stool guaiac*, dry mouth, gastritis, stomatitis, vomiting.
Central Nervous System: Dizziness*, headache*, fatigue, increased sweating, insomnia, nervousness, somnolence.
Dermatologic: Pruritus*, rash*.
Special Senses: Tinnitus*. Miscellaneous: Edema*.
*Incidence of reported reaction between 3% and 9%. Reactions occurring in 1% to 3% of the patients are unmarked.
Gastrointestinal: Anorexia, jaundice, duodenal ulcer, dysphagia, gastric ulcer, gastroenteritis, gastrointestinal bleeding, increased appetite, liver function abnormalities, melena, hepatic failure.
Central Nervous System: Asthenia, agitation, anxiety, confusion, depression, malaise, paresthesia, tremor, vertigo.
Dermatologic: Bullous eruptions, photosensitivity, urticaria, pseudoporphyria cutanea tarda, toxic epidermal necrolysis, erythema multiforme, Stevens-Johnson syndrome.
Cardiovascular: Vasculitis.
Metabolic: Weight gain.
Respiratory: Dyspnea, eosinophilic pneumonia, hypersensitivity pneumonitis, idiopathic interstitial pneumonitis.
Genitourinary: Albuminuria, azotemia, hyperuricemia, interstitial nephritis, nephrotic syndrome, vaginal bleeding, renal failure.
Special Senses: Abnormal vision.
Hematologic/Lymphatic: Thrombocytopenia.
Hypersensitivity: Anaphylactoid reaction, anaphylaxis, angioneurotic edema.
†Adverse reactions reported only in worldwide postmarketing experience or in the literature, not seen in clinical trials, are considered rarer and are italicized.
Gastrointestinal: Bilirubinuria, duodenitis, eructation, gallstones, gingivitis, glossitis, pancreatitis, rectal bleeding.
Central Nervous System: Nightmares.
Dermatologic: Acne, alopecia.
Cardiovascular: Angina, arrhythmia, hypertension, myocardial infarction, palpitations, syncope, thrombophlebitis.
Respiratory: Asthma, cough.
Genitourinary: Dysuria, hematuria, impotence, renal stones.
Special Senses: Taste disorder.
Body as a Whole: Fever, chills.
Hematologic/Lymphatic: Anemia, leukopenia, granulocytopenia.
Metabolic/Nutritional: Hyperglycemia, hypokalemia, weight loss.
What drugs interact with Relafen (nabumetone)?
- ACE-inhibitors: Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.
- Aspirin: When Relafen (nabumetone) is administered with aspirin, its protein binding is reduced, although the clearance of free Relafen (nabumetone) is not altered. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of nabumetone and aspirin is not generally recommended because of the potential of increased adverse effects.
- Diuretics: Clinical studies, as well as post marketing observations, have shown that Relafen (nabumetone) can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure, as well as to assure diuretic efficacy.
- Lithium: NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
- Methotrexate: NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
- Warfarin: The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
- In vitro studies have shown that, because of its affinity for protein, 6MNA may displace other protein-bound drugs from their binding site. Caution should be exercised when administering Relafen (nabumetone) with warfarin since interactions have been seen with other NSAIDs.
- Concomitant administration of an aluminum-containing antacid had no significant effect on the bioavailability of 6MNA. When administered with food or milk, there is more rapid absorption; however, the total amount of 6MNA in the plasma is unchanged.
Summary
Relafen (nabumetone) is a nonsteroidal anti-inflammatory drug (NSAID) used to treat inflammation and pain from rheumatoid arthritis and osteoarthritis. Common side effects of Relafen include abdominal pain, cramping, nausea, gastritis, rash, and ringing in the ears. In late pregnancy, as with other NSAIDs, Relafen should be avoided because it may cause premature closure of the ductus arteriosus. It is unknown if Relafen is excreted in breast milk. Consult your doctor before breastfeeding.


