Contents
Side Effects of Daypro (oxaprozin)
NSAIDs reduce levels of prostaglandins, chemicals that cause pain, fever, and inflammation. Daypro blocks the enzyme that makes prostaglandins (cyclooxygenase), reducing concentrations of prostaglandins and therefore reducing inflammation, pain, and fever.
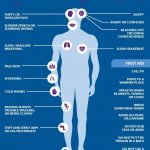
Common side effects of Daypro include a rash, ringing in the ears, headaches, dizziness, drowsiness, abdominal pain, nausea, diarrhea, constipation, heartburn, fluid retention, and shortness of breath.
Serious side effects of Daypro include increased bleeding after an injury, bleeding in the stomach and intestine, ulcers (signs include black tarry stools, weakness, and dizziness upon standing), impaired kidney function, blood clots, heart attacks, high blood pressure, and heart failure.
Drug interactions of Daypro include lithium, which can increase the blood levels of lithium and lead to toxicity. Daypro can also reduce the effectiveness of blood pressure medications. When used with methotrexate or aminoglycoside antibiotics, Daypro can increase blood levels of those drugs and potentially cause more side effects. Daypro can also increase the negative effects of cyclosporine on kidney function. Taking aspirin with Daypro may increase the risk of developing an ulcer. Individuals who consume more than 3 alcoholic beverages per day may have an increased risk of developing stomach ulcers when taking Daypro or other NSAIDs.
Taking Daypro during the last three months of pregnancy may harm the fetus and interfere with normal labor and delivery. Consult your doctor if you are pregnant or plan to become pregnant. It is unknown if Daypro is excreted in breast milk. Consult your doctor before breastfeeding.
What are the important side effects of Daypro (oxaprozin)?
The most common side effects of oxaprozin include a rash, ringing in the ears, headaches, dizziness, drowsiness, abdominal pain, nausea, diarrhea, constipation, heartburn, fluid retention, and shortness of breath. NSAIDs reduce the ability of blood to clot and may cause bleeding in the stomach and intestine, as well as ulcers.
Stomach ulceration and intestinal bleeding can occur without abdominal pain. Black tarry stools, weakness, and dizziness upon standing may be the only signs of bleeding.
People who are allergic to other NSAIDs should not use oxaprozin. NSAIDs reduce blood flow to the kidneys and impair kidney function, which is more likely to occur in patients with preexisting kidney impairment or congestive heart failure.
Individuals with asthma are more likely to experience allergic reactions to oxaprozin and other NSAIDs. NSAIDs have been associated with fluid retention, blood clots, heart attacks, hypertension, and heart failure.
Daypro (oxaprozin) side effects list for healthcare professionals
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events
- GI Bleeding, Ulceration and Perforation
- Hepatotoxicity
- Hypertension
- Heart Failure and Edema
- Renal Toxicity and Hyperkalemia
- Anaphylactic Reactions
- Serious Skin Reactions
- Hematologic Toxicity
Clinical Trials Experience
Adverse reaction data were derived from patients who received Daypro in multidose, controlled, and open-label clinical trials. Rates for events from clinical trial experience are based on 2253 patients who took 1200 mg to 1800 mg Daypro per day.
Incidence Greater Than 1%
In clinical trials of Daypro, the following adverse reactions occurred at an incidence greater than 1%:
Digestive system: abdominal pain/distress, anorexia, constipation, diarrhea, dyspepsia, flatulence, gastrointestinal ulcers, bleeding/perforation, heartburn, liver enzyme elevations, nausea, vomiting.
Hematologic system: anemia, increased bleeding time.
Nervous system: CNS inhibition (depression, sedation, somnolence, confusion), sleep disturbance, dizziness, headache.
Skin and appendages: pruritus, rash.
Urogenital system: abnormal renal function, dysuria, frequency.
Incidence Greater Than 1%
The following adverse reactions were reported in clinical trials:
Body as a whole: appetite changes, death, drug hypersensitivity reactions including anaphylaxis, fever, infection, sepsis.
Cardiovascular system: arrhythmia, blood pressure changes, congestive heart failure, hypertension, hypotension, myocardial infarction, palpitations, tachycardia, syncope, vasculitis.
Digestive system: alteration in taste, dry mouth, eructation, esophagitis, gastritis, glossitis, hematemesis, jaundice, liver function abnormalities including liver failure, stomatitis, hemorrhoidal or rectal bleeding.
Hematologic system: aplastic anemia, ecchymoses, eosinophilia, hemolytic anemia, lymphadenopathy, melena, purpura, thrombocytopenia, leukopenia. Metabolic system: hyperglycemia, weight changes.
Nervous system: anxiety, asthenia, coma, convulsions, dream abnormalities, drowsiness, hallucinations, insomnia, malaise, meningitis, nervousness, paresthesia, tremors, vertigo, weakness.
Respiratory system: asthma, dyspnea, pulmonary infections, pneumonia, sinusitis, upper respiratory tract infection, respiratory depression.
Urogenital: cystitis, hematuria, increase in menstrual flow, oliguria/polyuria, proteinuria, renal insufficiency, decreased menstrual flow.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Daypro:
Hematologic system: agranulocytosis, pancytopenia.
Skin: pseudoporphyria, exfoliative dermatitis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis.
Urogenital: acute interstitial nephritis, nephrotic syndrome, acute renal failure.
What drugs interact with Daypro (oxaprozin)?
See Table 2 for clinically significant drug interactions with oxaprozin.
Table 2: Clinically Significant Drug Interactions with Oxaprozin
- Oxaprozin and anticoagulants such as warfarin have a synergistic effect on bleeding.
- Serotonin reuptake inhibitors and NSAIDs may potentiate the risk of bleeding.
Daypro is not a substitute for low-dose aspirin for cardiovascular protection.
- NSAIDs may diminish the antihypertensive effect of ACE inhibitors, ARBs, or beta-blockers.
- In patients who are elderly, volume-depleted, or have renal impairment, co-administration of an NSAID with ACE inhibitors or ARBs may result in deterioration of renal function.
- During concomitant use of Daypro and ACE inhibitors, ARBs, or beta-blockers, monitor blood pressure.
- During concomitant use of Daypro and ACE inhibitors or ARBs in patients who are elderly, volume-depleted, or have impaired renal function, monitor signs of worsening renal function.
- When these drugs are administered concomitantly, patients should be adequately hydrated and renal function should be assessed.
Avoid using NSAIDs with short elimination half-lives before and after administration of pemetrexed.
Laboratory Test Interactions
False-positive urine immunoassay screening tests for benzodiazepines have been reported in patients taking Daypro. Confirmatory tests, such as gas chromatography/mass spectrometry, will distinguish Daypro from benzodiazepines.
Summary
Daypro (oxaprozin) is a nonsteroidal anti-inflammatory drug (NSAID) used to relieve the signs and symptoms of osteoarthritis, rheumatoid arthritis, and juvenile rheumatoid arthritis. Common side effects include a rash, ringing in the ears, headaches, dizziness, drowsiness, abdominal pain, nausea, diarrhea, constipation, heartburn, fluid retention, and shortness of breath. Taking Daypro during pregnancy or while breastfeeding may have negative effects. Consult your doctor for further guidance.