Contents
Omaveloxolone: A Novel Treatment for Friedreich’s Ataxia
Omaveloxolone is an oral medication approved by the FDA in February 2023 for the treatment of Friedreich’s ataxia, a genetic neurodegenerative disorder. This disorder affects the nervous system and impairs movement, balance, and speech. Omaveloxolone is expected to be commercially available in the second quarter of 2023.
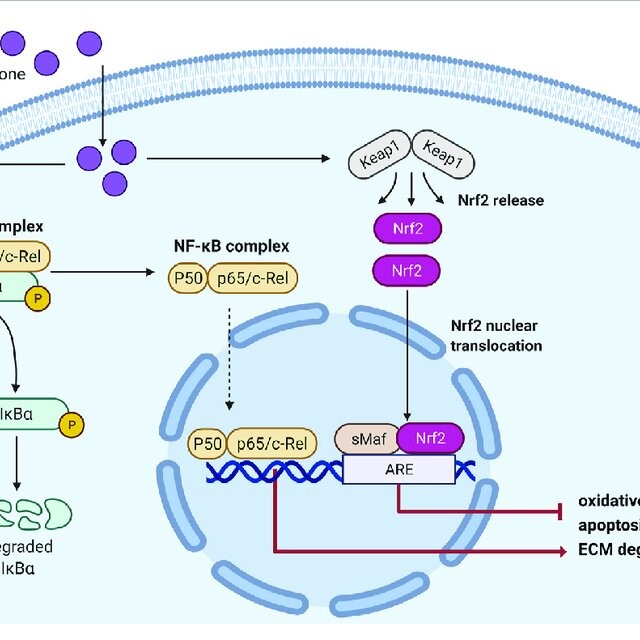
Belonging to a class of medications known as Nrf2 activators, omaveloxolone works by reducing inflammation and oxidative stress caused by free radicals. Free radicals, also known as reactive oxygen species (ROS), can cause tissue damage. Omaveloxolone activates Nrf2, which stimulates pathways that resolve inflammation, inhibit pro-inflammatory signaling, and restore mitochondrial function. These pathways are usually impaired in Friedreich’s ataxia.
Warnings
- Omaveloxolone may cause elevation of liver enzymes. Use with caution in patients with pre-existing liver impairment. Monitor liver function regularly.
Side Effects
Common side effects of omaveloxolone include:
- Elevated liver enzymes ALT and AST
- Headache
- Nausea
- Vomiting
- Abdominal pain
- Diarrhea
- Reduced appetite
- Fatigue
- Musculoskeletal pain
- Mouth and throat (oropharyngeal) pain
- Muscle spasms
- Back pain
- Influenza
- Rash
- Increase in LDL cholesterol
- Decrease in HDL cholesterol
- Elevation of brain-type natriuretic peptide (BNP)
If you experience any serious side effects such as heart symptoms, severe headache, nervous system reaction, or serious eye symptoms, seek medical attention immediately.
Dosages
Adults and adolescents aged 16 years and above with Friedreich’s ataxia should take 150 mg (3 capsules) of omaveloxolone orally every day. Dosage modifications may be required for patients with renal impairment. Avoid coadministration with certain inhibitors and moderate or strong inducers.
Overdose
The effects of omaveloxolone overdose are not known. Overdose may worsen adverse reactions such as abnormalities in cholesterol levels and elevation of liver enzymes and BNP. Treatment should focus on managing symptoms and providing supportive care.
Drug Interactions
Inform your doctor of all medications you are taking to prevent any potential drug interactions. Omaveloxolone has serious or moderate interactions with various drugs.
Pregnancy and Breastfeeding
- Omaveloxolone may cause fetal harm. Its safety in pregnant women has not been established.
- Omaveloxolone may reduce the efficacy of hormonal contraceptives. Additional non-hormonal contraceptives should be used during treatment and for 28 days after the final dose.
- The effects of omaveloxolone on breastfeeding mothers and infants are unknown. The decision to breastfeed should consider the mother’s clinical need for the medication and the potential risk to the infant.
Additional Information
- Take omaveloxolone as prescribed and undergo periodic blood tests. Report any symptoms of fluid overload and heart failure to your physician.
- Discuss any medication changes with your doctor or healthcare provider.
Summary
Omaveloxolone is a novel oral medication that treats Friedreich’s ataxia, a genetic neurodegenerative disorder. Common side effects include elevated liver enzymes, headache, nausea, vomiting, abdominal pain, and muscle spasms.