Contents
leuprolide – injection, Lupron
This site displays drug information subject to the terms of use. By continuing to view the drug information, you agree to abide by these terms of use.
GENERIC NAME: LEUPROLIDE – INJECTION (LOO-proe-lide)
BRAND NAME(S): Lupron
USES: Leuprolide is used to treat advanced prostate cancer in men. Most types of prostate cancer need testosterone to grow and spread. Leuprolide reduces the amount of testosterone that the body makes, helping to slow or stop cancer cell growth and relieve symptoms like painful/difficult urination. Discuss treatment risks and benefits with your doctor. Leuprolide is also used to stop early puberty (precocious puberty) in children. It delays sexual development (e.g., growth of breasts/testicles) and the start of menstrual periods, and slows down early bone growth to increase chances of reaching normal adult height. Leuprolide decreases the amount of sex hormones a child’s body makes (estrogen in girls, testosterone in boys).OTHER This section contains uses of this drug not listed in the approved professional labeling but may be prescribed by your healthcare professional. Use this drug for a condition listed in this section only if prescribed. Other leuprolide products may also be used to treat uterus disorders (e.g., endometriosis, fibroids). In females, leuprolide reduces the body’s estrogen production.
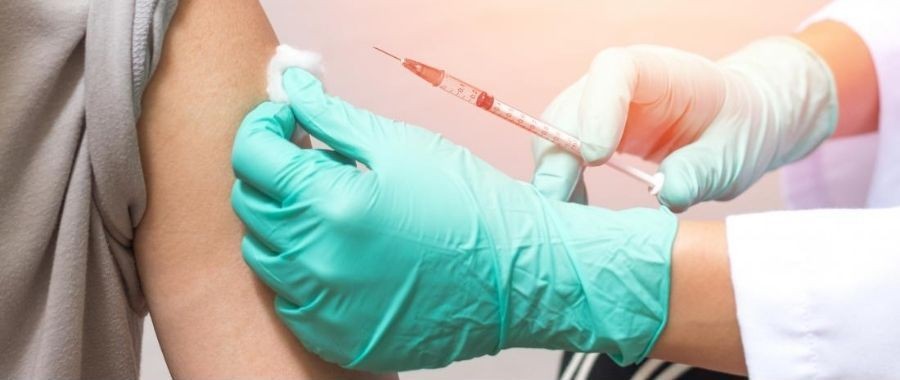
HOW TO USE: This medication is given as a subcutaneous injection, usually once a day or as directed by your doctor. In children, dosage is based on weight and response to therapy. The doctor should consider stopping treatment before age 11 for girls and age 12 for boys. Consult the doctor for details. If you are directed to inject this medication yourself, learn preparation and usage instructions in the product package. Learn how to store and discard needles and medical supplies safely. If any information is unclear, consult your doctor or pharmacist. Before using, visually check this product for particles or discoloration. If present, do not use the liquid. Change the injection site location each time to avoid problem areas under the skin. Use this medication regularly at the same time each day. Inform your doctor if symptoms do not improve or worsen.
SIDE EFFECTS: Mild burning/pain/bruising at the injection site, hot flashes (flushing), increased sweating, night sweats, tiredness, headache, upset stomach, breast changes, acne, joint/muscle aches, trouble sleeping, reduced sexual interest, vaginal discomfort/dryness, vaginal bleeding, swelling of the ankles/feet, increased urination at night, or dizziness may occur. If these effects persist or worsen, tell your doctor or pharmacist promptly. In men, shrinking of the testicles, breast tenderness/swelling, and reduced sexual interest/ability may occur due to lowered testosterone levels. Talk to your doctor if these effects occur. In girls, regular periods are expected to stop (or decrease to light bleeding/spotting during the first 2 months) with regular use of this medication. Tell your doctor promptly if regular periods continue after 2 months of treatment. Remember that your doctor has prescribed this medication because the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects. During the first few weeks of treatment, hormone levels may increase before they decrease. This is a normal response by your body to the drug and can result in a temporary increase in symptoms. In men, an increase in testosterone levels at the beginning of treatment may cause new or worsening symptoms for a few weeks. If you have prostate cancer that has spread to the spine or caused urinary blockage, you may require closer monitoring. Tell your doctor immediately if you experience any of the following serious side effects: bone pain, numbness/tingling/weakness of the arms/legs, blood in the urine, painful/difficult urination, unusual weakness, inability to move. Tell your doctor right away if you have any serious side effects, including: mental/mood changes (e.g., depression, thoughts of suicide, mood swings, aggression in children), new/worsening bone pain (in adults), easily broken bones (in adults), increased thirst/urination. Get medical help right away if you have any very serious side effects, including: chest/jaw/left arm pain, irregular heartbeat, weakness on one side of the body, slurred speech, seizures. Rarely, a very serious problem with your pituitary gland (pituitary apoplexy) may occur, usually in the first hour to 2 weeks after your first injection. Get medical help right away if any of these very serious side effects occur: sudden severe headache, sudden severe mental/mood changes (e.g., severe confusion, difficulty concentrating), vision changes, severe vomiting, fainting. A serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing. This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist. In the US, call your doctor for medical advice about side effects. You may report side effects to FDA. In Canada, call your doctor for medical advice about side effects. You may report side effects to Health Canada.
PRECAUTIONS: Before using leuprolide, tell your doctor or pharmacist if you are allergic to it or if you have any other allergies. This product may contain inactive ingredients (such as the preservative benzyl alcohol), which can cause allergic reactions or other problems. Talk to your pharmacist for more details. Before using this medication, tell your doctor or pharmacist your medical history, especially of: diabetes, heart disease (such as heart attack), stroke, high cholesterol, family history of sudden cardiac death. In adults, leuprolide may weaken bones and increase risk for bone loss (osteoporosis) if used long-term. Before using, tell your doctor or pharmacist if you have osteoporosis or any risk factors for osteoporosis (long-term alcohol use, smoking, family history of osteoporosis and broken bones, use of certain medications). This drug may make you dizzy. Do not drive, use machinery, or do any activity that requires alertness until you are sure you can perform safely. Limit alcoholic beverages. Leuprolide must not be used during pregnancy as it may harm an unborn baby. If you become pregnant or think you may be pregnant, inform your doctor immediately. Consult your doctor for more details and to discuss reliable forms of birth control. Non-hormonal methods (e.g., condoms, diaphragm with spermicide) are recommended during treatment with leuprolide. It is not known if leuprolide passes into breast milk. Because the effects of leuprolide on a nursing infant are unknown, breast-feeding is not recommended. Consult your doctor before breast-feeding.
DRUG INTERACTIONS: Your doctor or pharmacist may already be aware of possible drug interactions and may be monitoring you for them. Do not start, stop, or change the dosage of any medicine before checking with your doctor or pharmacist. Before using this medication, tell your doctor or pharmacist about all prescription and nonprescription/herbal products you use. Keep a list of all your medications and share it with your doctor and pharmacist.
OVERDOSE: If overdose is suspected, contact a poison control center or emergency room immediately. US residents can call their local poison control center. Canada residents can call a provincial poison control center.
NOTES: Do not share this medication with others. Periodic lab and/or medical tests (e.g., hormone levels, PSA blood test for men, bone tests, blood glucose) should be done to monitor progress. Consult your doctor for more details.
MISSED DOSE: Do not miss any doses. If you miss a dose, use it as soon as you remember. If it is near the time of the next dose, skip the missed dose and resume your usual schedule. Do not double the dose to catch up.
STORAGE: Different brands of this medication have different storage needs. Check the product package for instructions, or ask your pharmacist. Protect from light and freezing. Keep all medications away from children and pets. Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company for more details on how to safely discard the product.
Information last revised December 2013. Copyright(c) 2013 First Databank, Inc.
IMAGES
Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.