lamotrigine – oral, Lamictal
Warning: Rarely, serious and fatal skin rashes have occurred while taking this medication. These rashes are more common in children under 16 than in adults. Rashes may be more likely if you start at too high a dose or increase your dose too quickly. They may also occur if you take this medication with certain other anti-seizure medications like valproic acid or divalproex. These rashes can occur anytime during use, but most serious rashes happen within 2 to 8 weeks of starting lamotrigine. Get medical help right away if you develop any type of skin rash while taking this medication or if you have other signs of a serious allergic reaction such as hives, fever, swollen lymph glands, painful sores in the mouth or around the eyes, or swelling of the lips or tongue. Your doctor will tell you if you should stop taking lamotrigine. Even after you stop taking this medication, it is still possible for the rash to become life-threatening or cause permanent scars or other problems.
Uses: Lamotrigine is used alone or with other medications to prevent and control seizures. It may also help prevent extreme mood swings of bipolar disorder in adults. Lamotrigine is an anticonvulsant or antiepileptic drug that restores the balance of certain natural substances in the brain.
How to use: Read the Medication Guide and, if available, the Patient Information Leaflet provided by your pharmacist before starting lamotrigine and each time you get a refill. Take this medication by mouth with or without food as directed by your doctor. Swallow the tablets whole. Dosage is based on your medical condition, response to treatment, and use of certain interacting drugs. Follow your doctor’s dosing instructions exactly. Take this medication regularly to get the most benefit from it and to help you remember, take it at the same time(s) each day. Do not stop taking this medication without consulting your doctor. Some conditions may worsen when the drug is suddenly stopped. Your dose may need to be gradually decreased. Also, if you have stopped taking this medication, do not restart lamotrigine without consulting your doctor. Tell your doctor if your condition does not improve or worsens.
Side effects: Dizziness, drowsiness, headache, blurred/double vision, loss of coordination, shaking (tremor), nausea, vomiting, or upset stomach may occur. If any of these effects persist or worsen, tell your doctor or pharmacist promptly. A small number of people who take anticonvulsants for any condition may experience depression, suicidal thoughts/attempts, or other mental/mood problems. Tell your doctor right away if you or your family/caregiver notice any unusual/sudden changes in your mood, thoughts, or behavior including signs of depression or thoughts about harming yourself. Tell your doctor right away if any serious side effects occur: fainting, easy or unusual bruising/bleeding, unusual tiredness, signs of infection, muscle pain/tenderness/weakness, dark urine, yellowing eyes/skin, stomach/abdominal pain, persistent nausea/vomiting, change in the amount of urine. A serious allergic reaction to this drug is rare, but get medical help right away if you notice any symptoms of a serious allergic reaction such as rash, itching/swelling, severe dizziness, or trouble breathing. This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
Precautions: Before taking lamotrigine, tell your doctor or pharmacist if you are allergic to it or if you have any other allergies. This product may contain inactive ingredients that can cause allergic reactions or other problems. Talk to your pharmacist for more details. Before using this medication, tell your doctor or pharmacist your medical history, especially of kidney disease and liver disease. This drug may make you dizzy or drowsy or cause blurred vision. Do not drive, use machinery, or do any activity that requires alertness or clear vision until you are sure you can perform such activities safely. Limit alcoholic beverages. Before having surgery, tell your doctor or dentist about all the products you use. Older adults may be more sensitive to the side effects of this drug, especially dizziness, loss of coordination, or fainting. These side effects can increase the risk of falling. During pregnancy, this medication should be used only when clearly needed. It may harm an unborn baby. However, since untreated seizures are a serious condition that can harm both a pregnant woman and her unborn baby, do not stop taking this medication unless directed by your doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately talk to your doctor about the benefits and risks of using this medication during pregnancy. This drug passes into breast milk and may have undesirable effects on a nursing infant. Consult your doctor before breast-feeding.
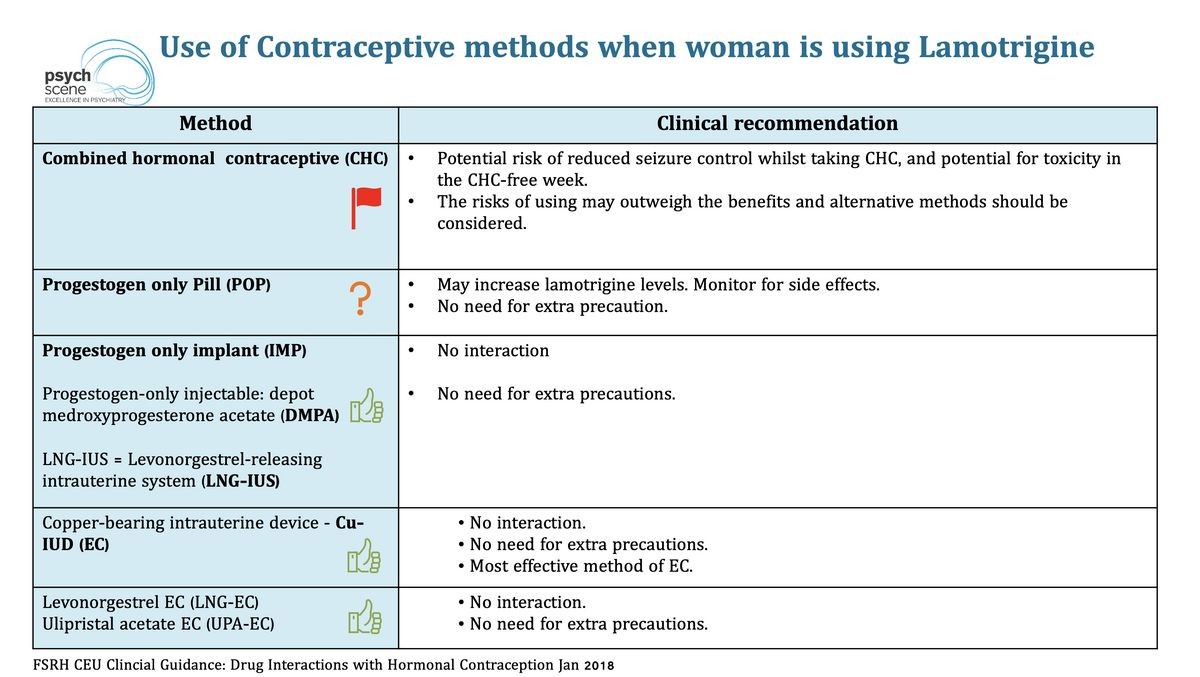
Drug interactions: Drug interactions may change how your medications work or increase your risk for serious side effects. Keep a list of all the products you use and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor’s approval. Other medications can affect the removal of lamotrigine from your body, which may affect how lamotrigine works. Your doctor may need to adjust your dose of lamotrigine if you are on these medications. This medication may decrease the effectiveness of hormonal birth control products. Tell your doctor or pharmacist if you are taking other products that cause drowsiness. Check the labels on all your medicines because they may contain ingredients that cause drowsiness. If overdose is suspected, contact a poison control center or emergency room immediately. Do not share this medication with others. Laboratory and/or medical tests may be performed periodically to monitor your progress or check for side effects. There are different types of this medication available. Some do not have the same effects. There are also some medications that sound the same as this product. Make sure you have the right product before taking it.
Missed dose: Take each dose at the scheduled time. If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose and resume your usual dosing schedule. Do not double the dose to catch up.
Storage: Store at room temperature away from light and moisture. Keep all medications away from children and pets. Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed.
Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.
Selected from data included with permission and copyrighted by First Databank, Inc. This material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
Conditions of use: The information in this database is intended to supplement the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate, or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet, or commencing or discontinuing any course of treatment.