Contents
Intelence
Intelence is used to treat human immunodeficiency virus type 1 (HIV-1) infection in antiretroviral treatment-experienced patients ages 2 years and older, who have viral replication and HIV-1 strains resistant to a non-nucleoside reverse transcriptase inhibitor (NNRTI) and other antiretroviral agents.
Side effects
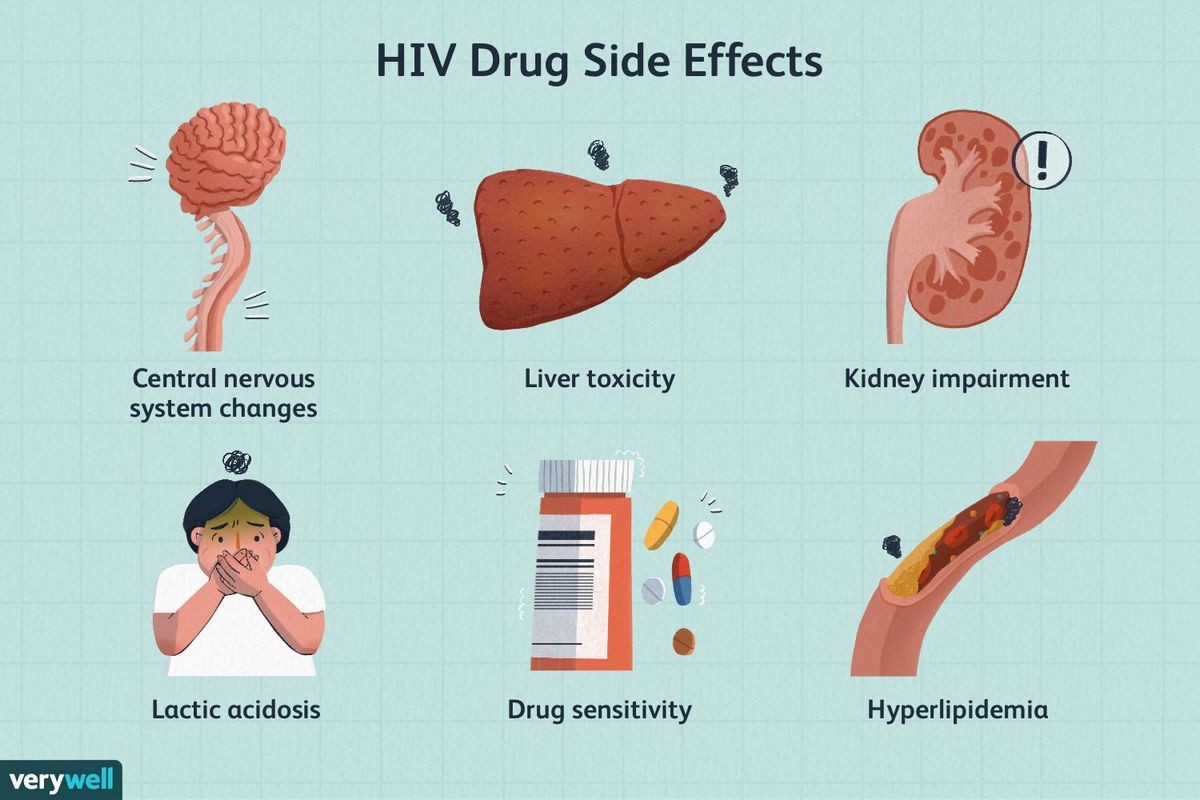
Intelence can cause serious side effects, including:
Severe skin rash and allergic reactions. Call your doctor right away if you get a rash with any of the following symptoms:
- hives or sores in your mouth, or your skin blisters and peels
- trouble swallowing or breathing
- swelling of your face, eyes, lips, tongue, or throat
- fever
- yellowing of the skin or whites of the eyes
- dark urine
- pain on the right side of the stomach-area (abdominal pain)
Changes in body fat can occur in people taking HIV medicines. These changes may include increased fat in the upper back and neck ("buffalo hump"), breast, and around the middle of the body (trunk). Loss of fat from the legs, arms, and face may also occur.
Changes in your immune system (Immune Reconstitution Syndrome) can occur when you start taking HIV medicines. Call your doctor right away if you start having any new symptoms after starting your HIV medicine. In adults, common side effects of Intelence include tingling, numbness, or pain in the hands or feet. In children, diarrhea is a common side effect.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects with Intelence.
Dosage
Adult Patients
The recommended oral dose of Intelence tablets is 200 mg (one 200 mg tablet or two 100 mg tablets) taken twice daily following a meal. The type of food does not affect the exposure to etravirine.
Pediatric Patients (2 Years to Less Than 18 Years of Age)
The recommended dose of Intelence for pediatric patients 2 years to less than 18 years of age and weighing at least 10 kg is based on body weight not exceeding the recommended adult dose. Intelence tablets should be taken orally, following a meal. The type of food does not affect the exposure to etravirine.
Drug interactions
Etravirine may interact with drugs that induce or inhibit certain enzymes, potentially altering the therapeutic effect or adverse reaction profile of Intelence. Etravirine itself may also interact with other drugs. Consult your doctor or pharmacist for more information.
The interaction between Intelence and certain drugs has been evaluated and no dose adjustment is needed for either drug.
Pregnancy and breastfeeding
Pregnancy
Use of Intelence during pregnancy may be justified if the potential benefit outweighs the potential risk to the fetus.
Antiretroviral Pregnancy Registry has been established to monitor maternal-fetal outcomes. Physicians are encouraged to register patients.
Reproductive and developmental toxicity studies in animals did not show any treatment-related effects or malformations.
Breastfeeding
HIV-infected mothers should not breastfeed to avoid the risk of postnatal transmission of HIV. It is not known whether etravirine is secreted in human milk.
Additional information
Intelence is a non-nucleoside reverse transcriptase inhibitor (NNRTI) used to treat HIV-1 infection. It is available in different tablet strengths. Etravirine works by blocking the enzyme responsible for HIV replication.
Intelence, in combination with other antiretroviral agents, is indicated for the treatment of HIV-1 infection in antiretroviral treatment-experienced patients ages 2 years and older, who have evidence of viral replication and HIV-1 strains resistant to a non-nucleoside reverse transcriptase inhibitor (NNRTI) and other antiretroviral agents.


