Contents
Ataluren
Ataluren is an investigational drug for delaying disease progression in ambulatory patients with Duchenne muscular dystrophy (DMD), a fatal neuromuscular disease. The medication is not yet FDA-approved or available in the U.S., but it has received conditional marketing authorization throughout the European Union (EU) since July 31, 2014. Currently, the drug is under additional monitoring by the European Medicines Agency (EMA) and can be found in some European countries, Brazil, Chile, Israel, and the Republic of Korea.
Duchenne muscular dystrophy is an X-linked genetic disease that primarily affects males and is inherited from carrier mothers. These carriers generally don’t exhibit symptoms or experience them mildly. DMD is a severe muscle wasting disease caused by the complete absence of dystrophin, a protein vital for muscle cell integrity, due to mutations in the dystrophin gene. This absence leads to progressive loss of skeletal muscle tissue, impairing mobility and eventually impacting the respiratory and cardiac muscles.
DMD symptoms usually manifest between the ages of 3 and 5, with patients typically becoming wheelchair-bound by age 14. Death occurs before the third decade due to cardiorespiratory complications. Becker muscular dystrophy (BMD), a rarer disease with milder symptoms and better life expectancy, is similar to DMD but less severe. Currently, there is no cure for either disease, and treatment involves the use of corticosteroids, cardiac medications, and assistive devices. Ataluren is the first drug targeting treatment by enabling the production of a modified dystrophin protein.
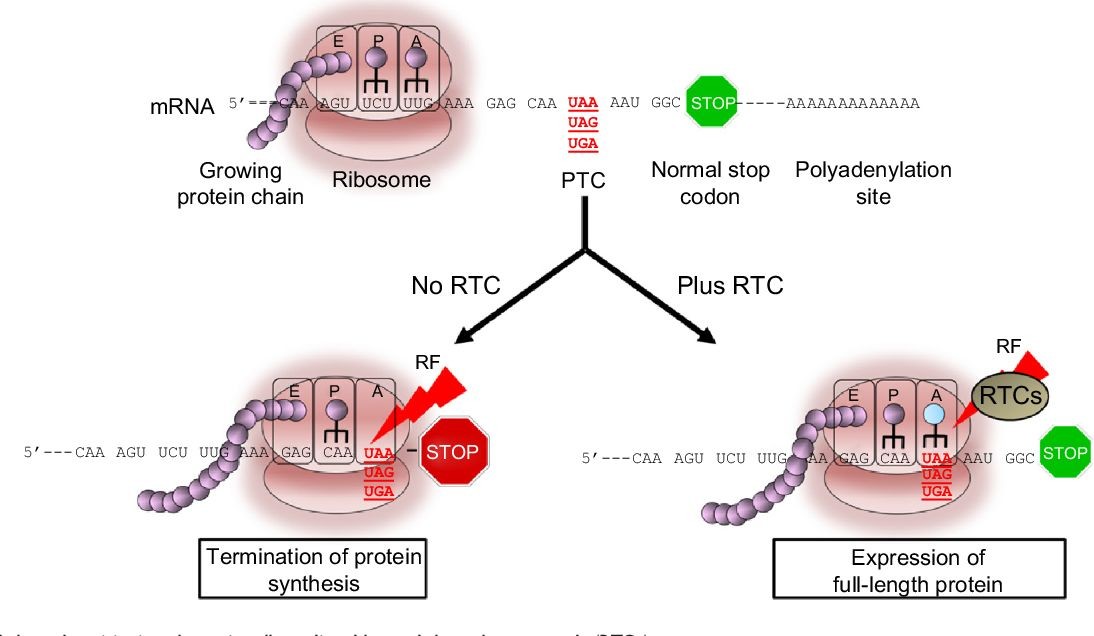
Ataluren is indicated for DMD resulting from a specific mutation known as nonsense mutation in the dystrophin gene, which is present in approximately 13% of DMD patients. Genetic testing determines the presence of this mutation. The mutation causes a premature ‘stop codon’ in the messenger RNA (mRNA), leading to an incomplete protein. Ataluren allows readthrough of the defective mRNA, preventing premature termination and facilitating the generation of a full-length, functional protein.
Warnings
- Avoid using ataluren if you have hypersensitivity to any of its components.
- Do not administer ataluren to DMD patients without the nonsense mutation of the dystrophin gene. Genetic testing is necessary before starting treatment.
- Avoid concurrent use of ataluren with intravenous (IV) aminoglycosides, a class of antibiotics that inhibits protein synthesis. Aminoglycosides reduce the effectiveness of ataluren and increase nephrotoxicity.
- Avoid concurrent use of ataluren with nephrotoxic drugs. Monitor kidney function closely if coadministration is unavoidable.
- Patients with severe renal impairment have higher exposure to ataluren and increased risk of toxicity from ataluren metabolite. Use ataluren in patients with severe renal impairment and end-stage renal disease only if the potential benefit outweighs the risk, closely monitor the patient, and consider reducing the dosage.
- Lipid profile changes have been reported in ataluren clinical trials. Monitor triglyceride and cholesterol levels annually, or more frequently if necessary.
- Corticosteroids used in conjunction with ataluren have caused hypertension in some patients. Monitor blood pressure every 6 months or more frequently if necessary.
- Ataluren has led to small increases in serum creatinine, blood urea nitrogen (BUN), and cystatin C in some patients. Monitor kidney function and relevant levels every 6 to 12 months or more frequently, depending on the patient’s clinical status.
QUESTION
What are the side effects of ataluren?
Common side effects of ataluren include:
- Headache
- Nausea
- Vomiting
- Diarrhea
- Upper abdominal pain
- Gas (flatulence)
- Abdominal discomfort
- Constipation
- Decrease in appetite
- Increase in cholesterol and triglyceride levels in blood
- Fever (pyrexia)
- Ear infection
- Red rash (erythematous rash)
- Feeling unwell (malaise)
- Weight loss
- High blood pressure (hypertension)
- Cough
- Nasal bleeding (epistaxis)
- Pain in extremity
- Musculoskeletal chest pain
- Urinary incontinence (enuresis)
- Blood in urine (hematuria)
- Increase in creatinine
- Increase in blood urea nitrogen (BUN)
- Increase in cystatin C
Call your doctor immediately if you experience any of the following symptoms or serious side effects:
- Fast or pounding heartbeats, fluttering in your chest, shortness of breath, and sudden dizziness (serious heart symptoms)
- Severe headache, confusion, slurred speech, severe weakness, vomiting, loss of coordination, feeling unsteady
- Very stiff muscles, high fever, sweating, confusion, fast or uneven heartbeats, tremors, feeling like you might pass out (severe nervous system reaction)
- Blurred vision, tunnel vision, eye pain or swelling, or seeing halos around lights (serious eye symptoms)
This is not an exhaustive list of side effects or adverse reactions. Consult your doctor for medical advice about serious side effects or adverse reactions. You can also report side effects or health problems to the FDA at 1-800-FDA-1088.
What are the dosages of ataluren?
Granules for oral suspension
Pediatric:
Duchenne Muscular Dystrophy (DMD)
- Indicated for treating DMD resulting from a nonsense mutation in the dystrophin gene in ambulatory patients aged 2 years (5 years in some countries) and older
- Mix granules with liquid or semi-solid food (e.g., yogurt) and administer orally
- Ataluren should be taken three times a day:
- 10 mg/kg in the morning
- 10 mg/kg at midday
- 20 mg/kg in the evening
- Total daily dose: 40 mg/kg
- No established safety and efficacy in children weighing less than 12 kg and aged below 2 years
Overdose
- Overdose with 200 mg/kg of ataluren in healthy individuals did not result in serious symptoms.
- Overdose symptoms included transient, low-grade nausea, vomiting, diarrhea, and headache.
- Treat ataluren overdose with symptomatic and supportive care.
What drugs interact with ataluren?
Inform your doctor of all medications you are currently taking to check for possible drug interactions. Never start, discontinue, or modify the dosage of any medication without your doctor’s recommendation.
Interactions with ataluren include:
The drug interactions mentioned above do not encompass all possible interactions or adverse effects. For more information, consult the RxList Drug Interaction Checker.
Always inform your doctor, pharmacist, or healthcare provider about all prescription and over-the-counter medications you use, including dosages. Maintain a list of this information. If you have any questions about your medication, consult your doctor or healthcare provider.
Pregnancy and breastfeeding
- No data on ataluren use in pregnant women; animal studies show fetal toxicity at maternally toxic doses
- Presence of ataluren in breast milk is unknown, but the drug and its metabolites have been found in animal milk. Avoid breastfeeding during ataluren therapy.
What else should I know about ataluren?
- Follow the prescribed dosage of ataluren.
- Stay adequately hydrated to prevent dehydration.
- Avoid driving or operating heavy machinery if you feel dizzy.
- Regularly attend lab tests as recommended by your physician.
- Keep ataluren out of reach of children.
- In case of overdose, contact your physician or Poison Control.
By clicking Submit, I agree to MedicineNet’s Terms & Conditions & Privacy Policy and understand that I may opt out of MedicineNet’s subscriptions at any time.
Summary
Ataluren is an investigational drug used to delay disease progression in ambulatory patients with Duchenne muscular dystrophy (DMD), a fatal neuromuscular disease. The medication is not yet FDA-approved or available in the U.S. Common side effects of ataluren include headache, nausea, vomiting, diarrhea, upper abdominal pain, gas (flatulence), abdominal discomfort, constipation, decrease in appetite, increase in cholesterol and triglyceride levels in blood, fever (pyrexia), ear infection, red rash (erythematous rash), feeling unwell (malaise), weight loss, high blood pressure (hypertension), and others.


