Contents
Adhansia XR (methylphenidate HCI)
Adhansia XR is a prescription medicine used to treat Attention Deficit Hyperactivity Disorder and Narcolepsy. It may be used alone or with other medications.
Adhansia XR belongs to the class of drugs called Stimulants; ADHD Agents.
The safety and effectiveness of Adhansia XR in children younger than 6 years of age is unknown.
Side Effects of Adhansia
ABUSE AND DEPENDENCE
CNS stimulants, including Adhansia XR, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence.
Adhansia XR may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- chest pain,
- trouble breathing,
- lightheadedness,
- hallucinations,
- new behavior problems,
- aggression,
- hostility,
- paranoia,
- numbness,
- pain,
- cold feeling,
- unexplained wounds,
- skin color changes (pale, red, or blue appearance) in your fingers or toes, and
- penis erection that is painful or lasts 4 hours or longer
Get medical help right away if you have any of the symptoms listed above.
The most common side effects of Adhansia XR include excessive swelling, mood changes, nervousness, irritability, sleep problems, fast heart rate, pounding heartbeats, fluttering in your chest, increased blood pressure, loss of appetite, weight loss, dry mouth, nausea, stomach pain, and headache.
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Adhansia XR. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Does Adhansia XR cause addiction or withdrawal symptoms?
Drug Abuse And Dependence
Controlled Substance
Adhansia XR contains methylphenidate, a Schedule II controlled substance.
Abuse
- CNS stimulants including Adhansia XR, other methylphenidate-containing products, and amphetamines have a high potential for abuse. Abuse is the intentional non-therapeutic use of a drug to achieve a desired effect. Abuse is characterized by impaired control over drug use, compulsive use, continued use despite harm, and craving.
- Signs and symptoms of CNS stimulant abuse include increased heart rate, respiratory rate, blood pressure, and sweating, dilated pupils, hyperactivity, restlessness, insomnia, decreased appetite, loss of coordination, tremors, flushed skin, vomiting, and abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed.
- To reduce the abuse of CNS stimulants including Adhansia XR, assess the risk of abuse prior to prescribing. After prescribing, keep careful prescription records, educate patients and their families about abuse, and monitor for signs of abuse.
Dependence
Tolerance
- Tolerance may occur during chronic therapy with CNS stimulants including Adhansia XR.
Dependence
- Physical dependence can occur in patients treated with CNS stimulants including Adhansia XR.
- Withdrawal symptoms after abrupt cessation of CNS stimulants include dysphoric mood, depression, fatigue, vivid dreams, insomnia or hypersomnia, increased appetite, and psychomotor retardation or agitation.
QUESTION
What is the dosage for Adhansia?
Pretreatment Screening
- Prior to initiating treatment with Adhansia XR, assess for the presence of cardiac disease and perform a careful history and physical exam.
- Assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence.
General Dosing Information
- Administer Adhansia XR orally once daily in the morning with or without food.
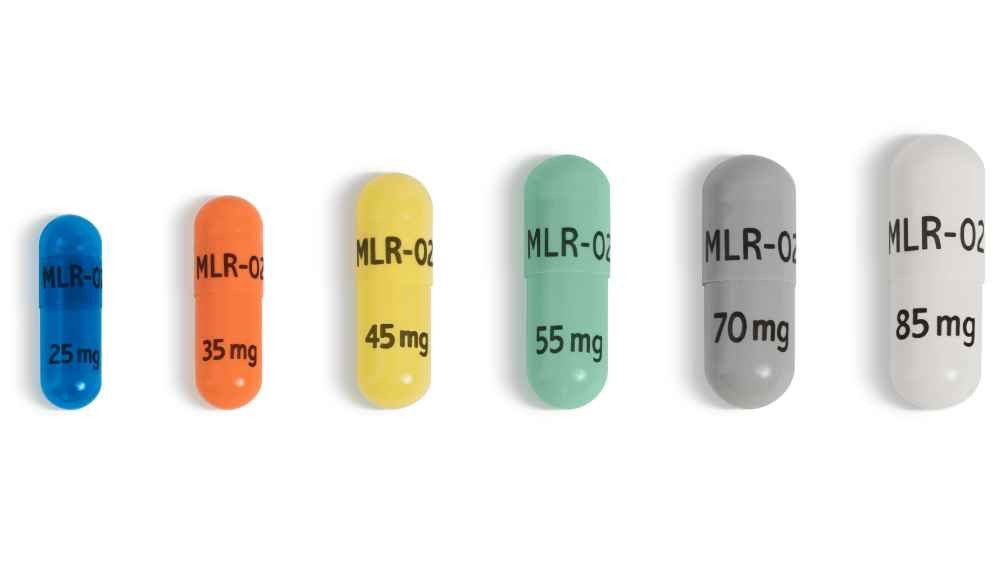
- The recommended starting dose of Adhansia XR for patients 6 years or older is 25 mg once daily. Titrate the dose in increments of 10 to 15 mg at intervals of no less than 5 days.
- Dosages higher than 100 mg daily in adults and 85 mg daily in pediatric patients have not been evaluated in clinical trials and are not recommended. Although efficacy was demonstrated in short-term controlled trials in adults at dosages of 100 mg daily, dosages above 85 mg daily were associated with a disproportionate increase in the incidence of certain adverse reactions.
- In short-term controlled trials in pediatric patients, efficacy was demonstrated at dosages of 70 mg daily, but dosages 70 mg daily and higher were associated with a disproportionate increase in the incidence of certain adverse reactions. Individualize dosage adjustments based upon assessment of clinical benefit and tolerability.
- Adhansia XR may be taken whole or the capsule may be opened and the entire contents sprinkled onto a tablespoon of applesauce or yogurt. The entire mixture should be consumed immediately or within 10 minutes.
- If the mixture is not consumed within 10 minutes after mixing, it should be discarded and not stored. Patients should take the entire contents of the capsule sprinkled on the chosen food in its entirety, without chewing. The dose of a single capsule should not be divided. Patients should not take anything less than one capsule per day.
- In the event of a missed dose, do not administer later in the day. Do not administer additional medication to make up for the missed dose.
- Periodically re-evaluate the long-term use of Adhansia XR and adjust dosage as needed.
Dose Reduction And Discontinuation
- If paradoxical aggravation of symptoms or other adverse reactions occur, reduce the dosage, or if necessary, discontinue the drug. Adhansia XR should be periodically discontinued to assess the patient’s condition. If improvement is not observed after appropriate dosage adjustment over a one-month period, discontinue Adhansia XR.
Switching From Other Methylphenidate Products
- If switching from other methylphenidate products, discontinue that treatment, and titrate with Adhansia XR using the titration schedule above.
- Do not substitute Adhansia XR for other methylphenidate products on a milligram-per-milligram basis because of different methylphenidate base compositions and differing pharmacokinetic profiles.
What drugs interact with Adhansia?
Clinically Important Drug Interactions
Table 3 presents clinically important drug interactions with Adhansia XR.
Table 3: Drugs Having Clinically Important Interactions with Adhansia XR
| Monoamine Oxidase Inhibitors (MAOI) | |
| Clinical Impact: | Concomitant use of MAOIs and CNS stimulants can cause hypertensive crisis. |
| Intervention: | Do not administer Adhansia XR concomitantly with MAOIs or within 14 days after discontinuing MAOI treatment. |
| Examples: | selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, methylene blue |
| Gastric pH Modulators | |
| Clinical Impact: | May change the release, PK profiles and alter the pharmacodynamics of Adhansia XR. |
| Intervention: | Monitor patients for changes in clinical effect and use alternative therapy based on clinical response. |
| Examples: | Omeprazole, esomeprazole, pantoprazole, famotidine, sodium bicarbonate |
Is Adhansia safe to use while pregnant or breastfeeding?
- Published studies and post-marketing reports on methylphenidate use during pregnancy are insufficient to identify a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
- Risks to the fetus associated with the use of CNS stimulants during pregnancy are unknown. Limited published literature reports that methylphenidate is present in human milk, but there are no reports of adverse effects on the breastfed infant and no effects on milk production.
- Long-term neurodevelopmental effects on infants from stimulant exposure are unknown. Consider the developmental and health benefits of breastfeeding along with the mother’s clinical need for Adhansia XR.
Summary
Adhansia XR (methylphenidate HCL) is a prescription medicine used to treat Attention Deficit Hyperactivity Disorder and Narcolepsy. It has a high potential for abuse and dependence. Serious side effects include hives, difficulty breathing, swelling of your face, lips, tongue, or throat; chest pain, trouble breathing, lightheadedness, hallucinations, new behavior problems, aggression, hostility, paranoia, and others.


