Contents
Side Effects of Minipress (prazosin)
Minipress (prazosin) is an alpha-1 adrenergic receptor blocker used to treat high blood pressure (hypertension).
By blocking alpha-1 receptors on muscle cells surrounding blood vessels, Minipress widens the blood vessels, decreasing blood flow resistance and ultimately lowering blood pressure.
Minipress seems to have a greater impact on reducing diastolic blood pressure, which corresponds to the minimum pressure in the arteries when the heart muscles are relaxed and the heart chambers are filling with blood.
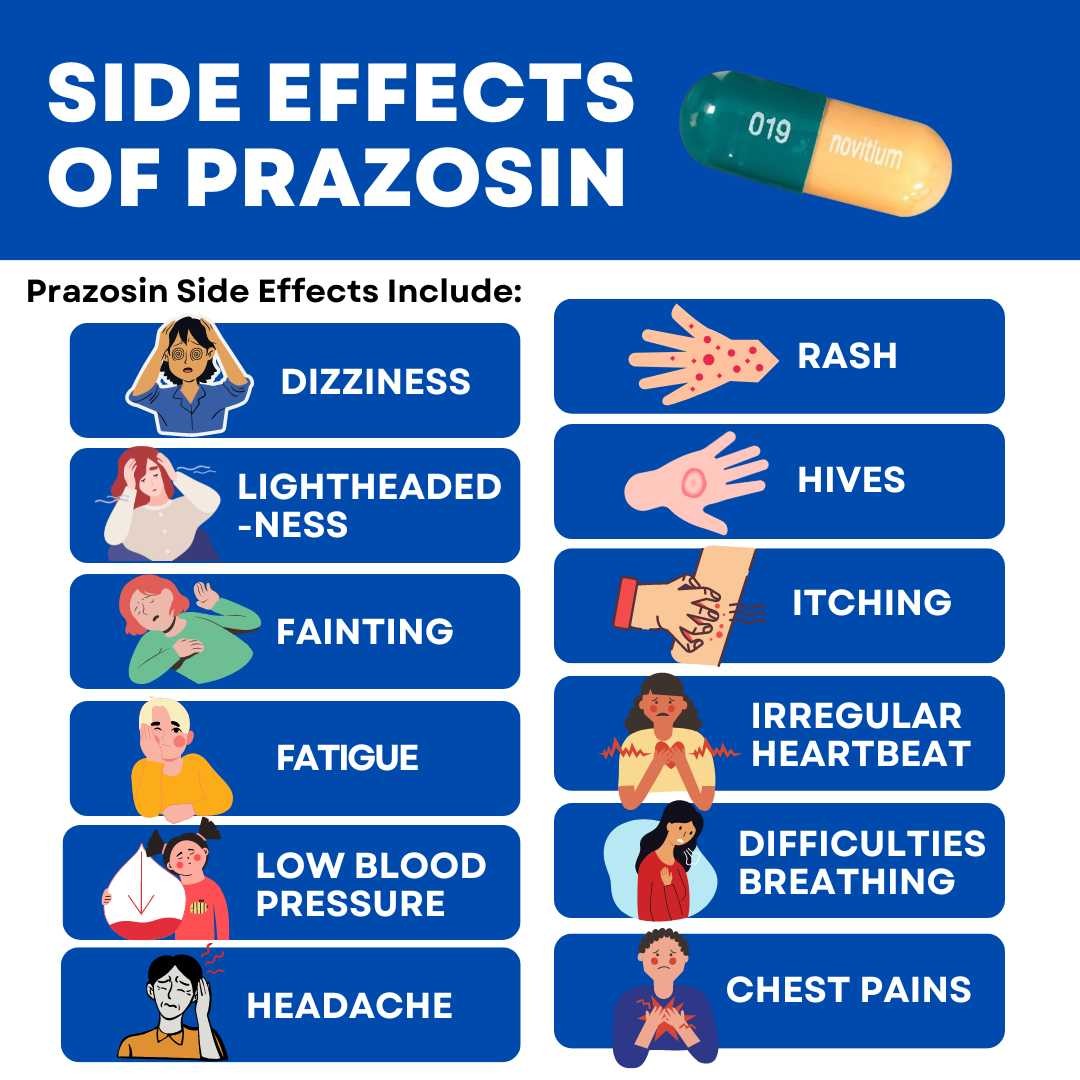
Common side effects of Minipress include:
Less common side effects of Minipress include:
- vomiting,
- diarrhea,
- constipation,
- fluid retention (edema),
- dizziness on standing,
- shortness of breath,
- fainting,
- motion sickness,
- depression,
- nervousness,
- rash,
- urinary frequency,
- blurred vision,
- eye redness,
- nosebleeds,
- dry mouth,
- nasal congestion.
Rare side effects of Minipress include:
- stomach pain,
- liver problems,
- pancreatitis,
- fast heartbeat,
- numbness/tingling/burning/prickling,
- hallucinations,
- itching,
- hair loss,
- urinary incontinence,
- sexual dysfunction,
- prolonged erection,
- ringing in the ears,
- sweating,
- fever,
- joint pain.
Minipress may interact with other blood pressure lowering medicines, water pills, or phosphodiesterase-5 (PDE-5) inhibitors, causing an additive decrease in blood pressure. Regarding pregnancy and breastfeeding, Minipress should only be used if the potential benefits outweigh the potential risks.
Important side effects of Minipress (prazosin)
The most common side effects of prazosin treatment include:
- dizziness,
- headache,
- drowsiness,
- lack of energy,
- weakness,
- palpitations, and
- nausea.
To minimize the chance of feeling dizzy or passing out, patients should:
- rise slowly from a sitting or lying position,
- climb stairs slowly,
- avoid alcohol, and
- drink plenty of water, especially in hot weather or during physical activity.
In addition, patients should have their blood pressure regularly checked.
Less common side effects include:
- vomiting,
- diarrhea,
- constipation,
- fluid retention (edema),
- dizziness on standing,
- shortness of breath,
- fainting (syncope),
- motion sickness,
- depression,
- nervousness,
- rash,
- urinary frequency,
- blurred vision,
- reddened sclera,
- nosebleeds,
- dry mouth,
- nasal congestion.
Rare side effects include:
- stomach pain,
- liver problems,
- pancreatitis,
- tachycardia (fast heartbeat),
- paresthesia (numbness, tingling, burning, prickling),
- hallucinations,
- itching,
- hair loss,
- urinary incontinence (loss of bladder control),
- sexual dysfunction,
- prolonged erection,
- ringing in the ears,
- sweating,
- fever,
- joint pain.
Other side effects reported in post-marketing trials include:
- allergic reaction,
- weakness,
- pain,
- chest pain,
- low blood pressure,
- gynecomastia (enlargement of men’s breasts),
- bradycardia (slow heartbeat),
- difficulty sleeping,
- hives,
- vasculitis,
- eye pain.
Minipress (prazosin) side effects list for healthcare professionals
During clinical trials and subsequent marketing experience, the most common reactions associated with Minipress therapy are:
- dizziness (10.3%),
- headache (7.8%),
- drowsiness (7.6%),
- lack of energy (6.9%),
- weakness (6.5%),
- palpitations (5.3%), and
- nausea (4.9%).
In most cases, side effects have resolved with continued therapy or have been tolerated with no dose reduction.
Less frequent adverse reactions reported in 1-4% of patients include:
Cardiovascular: edema, orthostatic hypotension, dyspnea, syncope.
Central Nervous System: vertigo, depression, nervousness.
Dermatologic: rash.
Genitourinary: urinary frequency.
EENT: blurred vision, reddened sclera, epistaxis, dry mouth, nasal congestion.
In addition, fewer than 1% of patients have reported the following (in some instances, exact causal relationships have not been established):
Gastrointestinal: abdominal discomfort and/or pain, liver function abnormalities, pancreatitis.
Cardiovascular: tachycardia.
Central Nervous System: paresthesia, hallucinations.
Genitourinary: incontinence, impotence, priapism.
Other: diaphoresis, fever, positive ANA titer, arthralgia.
Reports of pigmentary mottling, serous retinopathy, cataract development or disappearance, and worsening of pre-existing narcolepsy have been reported, although the exact causal relationship has not been established.
In post-marketing experience, the following adverse events have been reported:
Autonomic Nervous System: flushing.
Body As A Whole: allergic reaction, asthenia, malaise, pain.
Heart Rate/Rhythm: bradycardia.
Vascular (Extracardiac): vasculitis.
Vision: eye pain.
Special Senses: Intraoperative Floppy Iris Syndrome (IFIS) has been reported during cataract surgery in association with alpha-1 blocker therapy.
What drugs interact with Minipress (prazosin)?
Minipress has shown no adverse drug interactions in limited clinical experience with the following:
- cardiac glycosides-digitalis and digoxin;
- hypoglycemics-insulin, chlorpropamide, phenformin, tolazamide, and tolbutamide;
- tranquilizers and sedatives- chlordiazepoxide, diazepam, and phenobarbital;
- antigout-allopurinol, colchicine, and probenecid;
- antiarrhythmics-procainamide, propranolol, and quinidine; and
- analgesics, antipyretics, and anti-inflammatories-propoxyphene, aspirin, indomethacin, and phenylbutazone.
Adding a diuretic or other antihypertensive agent to Minipress can cause an additive hypotensive effect. This effect can be minimized by reducing the Minipress dose, introducing additional antihypertensive drugs cautiously, and retitrating Minipress based on clinical response.
Concomitant administration of Minipress with a phosphodiesterase-5 (PDE-5) inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension.
Drug/Laboratory Test Interactions
In a study on patients receiving prazosin, there was an average increase in the urinary metabolite of norepinephrine and an average increase in urinary VMA. Therefore, screening tests for pheochromocytoma may yield false-positive results in patients taking prazosin. If elevated VMA is found, prazosin should be discontinued and the patient retested after a month.
Laboratory Tests
In clinical studies, no adverse changes were noted between pre- and post-treatment lipid levels.
Summary
Minipress (prazosin) is an alpha-1 adrenergic receptor blocker used to treat high blood pressure (hypertension). Common side effects of Minipress include dizziness, headache, drowsiness, lack of energy, weakness, palpitations, and nausea. Less common side effects of Minipress include vomiting, diarrhea, constipation, fluid retention (edema), dizziness on standing, shortness of breath, fainting, motion sickness, depression, nervousness, rash, urinary frequency, blurred vision, eye redness, nosebleeds, dry mouth, and nasal congestion. Minipress should be used during pregnancy only if the potential benefit justifies the potential risk to the mother and the fetus. Minipress should be used cautiously in breastfeeding mothers.