Contents
Side Effects of Flonase (fluticasone)
Flonase (fluticasone) is a corticosteroid used to manage nasal symptoms of seasonal or perennial, allergic and non-allergic rhinitis in adults and children of 4 years of age and older.
The exact mechanism of action of Flonase is not known; however, it stimulates glucocorticoid receptors in humans that produces a potent anti-inflammatory response. Flonase also works on multiple cells and mediators that are responsible for the inflammatory symptoms of allergic rhinitis (sneezing, runny nose, etc.).
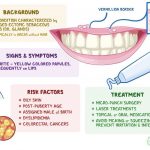
Common side effects of Flonase include headache, sore throat, nosebleeds, nasal burning or nasal irritation, nausea, vomiting, asthma symptoms, and cough.
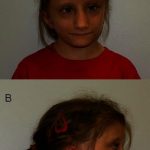
Serious side effects of Flonase include growth suppression from the use of inhaled steroids in some children.
Drug interactions of Flonase include ritonavir and ketoconazole, because they increase Flonase levels in the body by delaying its metabolism (elimination).
There are no adequate studies of Flonase to determine its safety and effectiveness in pregnant women. It is unknown if Flonase enters breast milk; therefore, it is best to be cautious before using it in breastfeeding mothers.
What are the important side effects of Flonase (fluticasone)?
Side effects of fluticasone include headache, sore throat, nosebleeds, nasal burning or nasal irritation, nausea, vomiting, asthma symptoms, or cough.
Flonase (fluticasone) side effects list for healthcare professionals
Systemic and local corticosteroid use may result in the following:
- Epistaxis, nasal ulceration, Candida albicans infection, nasal septal perforation, and impaired wound healing
- Cataracts and glaucoma
- Immunosuppression
- Hypercorticism and adrenal suppression
- Effect on growth
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In controlled US clinical trials, more than 3,300 subjects with allergic and nonallergic rhinitis received treatment with intranasal fluticasone propionate. In general, adverse reactions in clinical trials have been primarily associated with irritation of the nasal mucous membranes, and the adverse reactions were reported with approximately the same frequency by subjects treated with placebo.
- Less than 2% of subjects in clinical trials discontinued because of adverse reactions; this rate was similar for vehicle placebo and active comparators.
- The safety data described below are based on 7 placebo-controlled clinical trials in subjects with allergic rhinitis.
- The 7 trials included 536 subjects treated with Flonase 200 mcg once daily over 2 to 4 weeks and 2 placebo-controlled clinical trials which included 246 subjects treated with Flonase 200 mcg once daily over 6 months (Table 1).
- Also included in Table 1 are adverse reactions from 2 trials in which 167 children were treated with Flonase 100 mcg once daily for 2 to 4 weeks.
Table 1: Adverse Reactions with Flonase Nasal Spray with > 3% Incidence and More Common than Placebo in Subjects ≥ 4 Years with Allergic Rhinitis
| Adverse Reaction | Flonase 100 mcg Once Daily (n = 167) % |
Flonase 200 mcg Once Daily (n = 782) % |
Placebo (n = 758) % |
| Headache | 6.6 | 16.1 | 14.6 |
| Pharyngitis | 6.0 | 7.8 | 7.2 |
| Epistaxis | 6.0 | 6.9 | 5.4 |
| Nasal burning/nasal irritation | 2.4 | 3.2 | 2.6 |
| Nausea/vomiting | 4.8 | 2.6 | 2.0 |
| Asthma symptoms | 7.2 | 3.3 | 2.9 |
| Cough | 3.6 | 3.8 | 2.8 |
Other adverse reactions with Flonase Nasal Spray observed with an incidence less than or equal to 3% but greater than or equal to 1% and more common than with placebo included:
- blood in nasal mucus,
- runny nose,
- abdominal pain,
- diarrhea,
- fever,
- flu-like symptoms,
- aches and pains,
- dizziness, and
- bronchitis.
Postmarketing Experience
In addition to adverse events reported from clinical trials, the following adverse events have been identified during postapproval use of intranasal fluticasone propionate.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate or a combination of these factors.
General Disorders and Administration Site Conditions
- Hypersensitivity reactions, including angioedema, skin rash, edema of the face and tongue, pruritus, urticaria, bronchospasm, wheezing, dyspnea, and anaphylaxis/anaphylactoid reactions, which in rare instances were severe.
Ear and Labyrinth Disorders
- Alteration or loss of sense of taste and/or smell and, rarely, nasal septal perforation, nasal ulcer, sore throat, throat irritation and dryness, cough, hoarseness, and voice changes.
Eye Disorders
- Dryness and irritation, conjunctivitis, blurred vision, glaucoma, increased intraocular pressure, and cataracts.
- Cases of growth suppression have been reported for intranasal corticosteroids, including Flonase.
What drugs interact with Flonase (fluticasone)?
Inhibitors Of Cytochrome P450 3A4
- Fluticasone propionate is a substrate of CYP3A4. The use of strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin, conivaptan, lopinavir, nefazodone, voriconazole) with Flonase Nasal Spray is not recommended because increased systemic corticosteroid adverse effects may occur.
- A drug interaction trial with fluticasone propionate aqueous nasal spray in healthy subjects has shown that ritonavir (a strong CYP3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations.
- During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate products, including Flonase, with ritonavir, resulting in systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression.
Ketoconazole
- Coadministration of orally inhaled fluticasone propionate (1,000 mcg) and ketoconazole (200 mg once daily) resulted in a 1.9-fold increase in plasma fluticasone propionate exposure and a 45% decrease in plasma cortisol area under the curve (AUC), but had no effect on urinary excretion of cortisol.
Summary
Flonase (fluticasone) is a corticosteroid used to manage nasal symptoms of seasonal or perennial, allergic and non-allergic rhinitis in adults and children of 4 years of age and older. Common side effects of Flonase include headache, sore throat, nosebleeds, nasal burning or nasal irritation, nausea, vomiting, asthma symptoms, and cough. There are no adequate studies of Flonase to determine its safety and effectiveness in pregnant women. It is unknown if Flonase enters breast milk; therefore, it is best to be cautious before using it in breastfeeding mothers.