Contents
Side Effects of Depo Provera (Medroxyprogesterone)
Depo-Provera (medroxyprogesterone) is a progestin used to treat amenorrhea, abnormal uterine bleeding, endometrial cancer, and renal cancer.
Progestins prepare the endometrium for implantation of the embryo and help maintain pregnancy. At high doses, progestins prevent ovulation and pregnancy.
Common side effects of Depo-Provera include:
- breast tenderness
- liquid leakage from the nipple
- skin reactions (hives, acne, hair growth, hair loss)
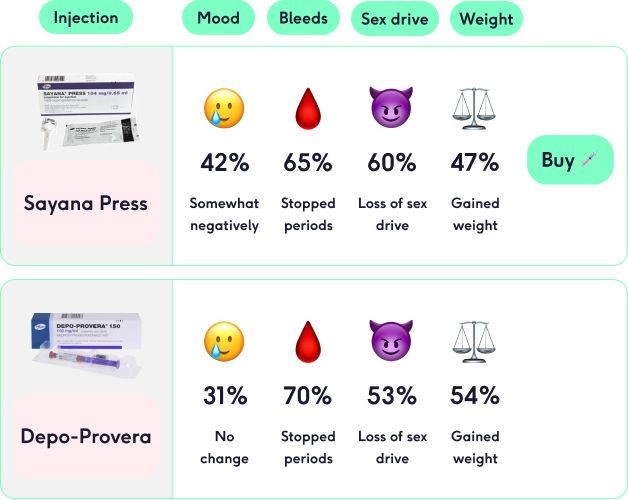
- break-through bleeding
- vaginal spotting of blood
- changes in menstrual flow
- changes in weight
- nausea
- fever
- insomnia
- yellowing skin and eyes (jaundice)
Serious side effects of Depo-Provera include blood clots and difficulty controlling blood glucose in diabetic patients.
Aminoglutethimide may decrease the effectiveness of Depo-Provera. It should not be used during pregnancy and may be secreted in breast milk.
Important Side Effects of Depo-Provera (Medroxyprogesterone)
Medroxyprogesterone may cause breast tenderness, liquid leakage from the nipple, skin reactions, break-through bleeding, changes in menstrual flow, weight changes, nausea, fever, insomnia, and jaundice.
Progestin therapy may increase the risk of blood clots, especially in smokers. It may also affect blood sugar control in diabetic patients.
The Women’s Health Initiative study found an increased risk of heart attacks, stroke, breast cancer, blood clots, and pulmonary emboli in postmenopausal women who took medroxyprogesterone. It is not recommended for heart disease or dementia prevention.
List of Side Effects of Depo-Provera (Medroxyprogesterone) for Healthcare Professionals
Depo-Provera may cause breakthrough bleeding, spotting, changes in menstrual flow, amenorrhea, changes in cervical erosion and secretions, breast tenderness, galactorrhea, and erectile dysfunction.
Other reported side effects include headaches, dizziness, somnolence, convulsions, nervousness, euphoria, mental depression, and insomnia.
Depo-Provera may also cause edema, pyrexia, fatigue, malaise, injection site reactions, injection site pain and tenderness, and injection site atrophy, indentation, dimpling, and lumps.
Other side effects include changes in weight, cholestatic jaundice, skin reactions, hypersensitivity reactions, angioedema, gastrointestinal disorders, corticoid-like effects, and alterations in laboratory test results.
Increased monitoring of blood sugar and adjustment of diabetes medications is recommended for diabetic patients taking medroxyprogesterone.
The use of Depo-Provera may alter laboratory test results related to liver function, coagulation, gonadotropins, sex-hormone binding globulin, and steroid levels.
Drug Interactions with Depo-Provera (Medroxyprogesterone)
Aminoglutethimide may decrease the effectiveness of Depo-Provera. Other drugs that may interact with Depo-Provera include strong CYP3A inhibitors and inducers.
Aminoglutethimide administered with Depo-Provera may decrease its concentration in the blood. Strong CYP3A inhibitors may increase the concentrations of medroxyprogesterone acetate, while strong CYP3A inducers may decrease its concentrations.
Patients should avoid coadministration with ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole, phenytoin, carbamazepine, rifampin, rifabutin, rifapentin, phenobarbital, and St. John’s Wort.
Progestin therapy may affect laboratory test results, so the pathologist should be informed when relevant specimens are submitted.
Depo-Provera contains a derivative of progesterone and is used to treat various conditions. Common side effects include breast tenderness, skin reactions, changes in menstrual flow, and weight changes. Serious side effects include blood clots and difficulty controlling blood glucose in diabetic patients. Aminoglutethimide may decrease its effectiveness, and drug interactions with strong CYP3A inhibitors and inducers should be avoided. Close monitoring and caution are advised when using Depo-Provera.