Contents
Side Effects of Corlanor (ivabradine)
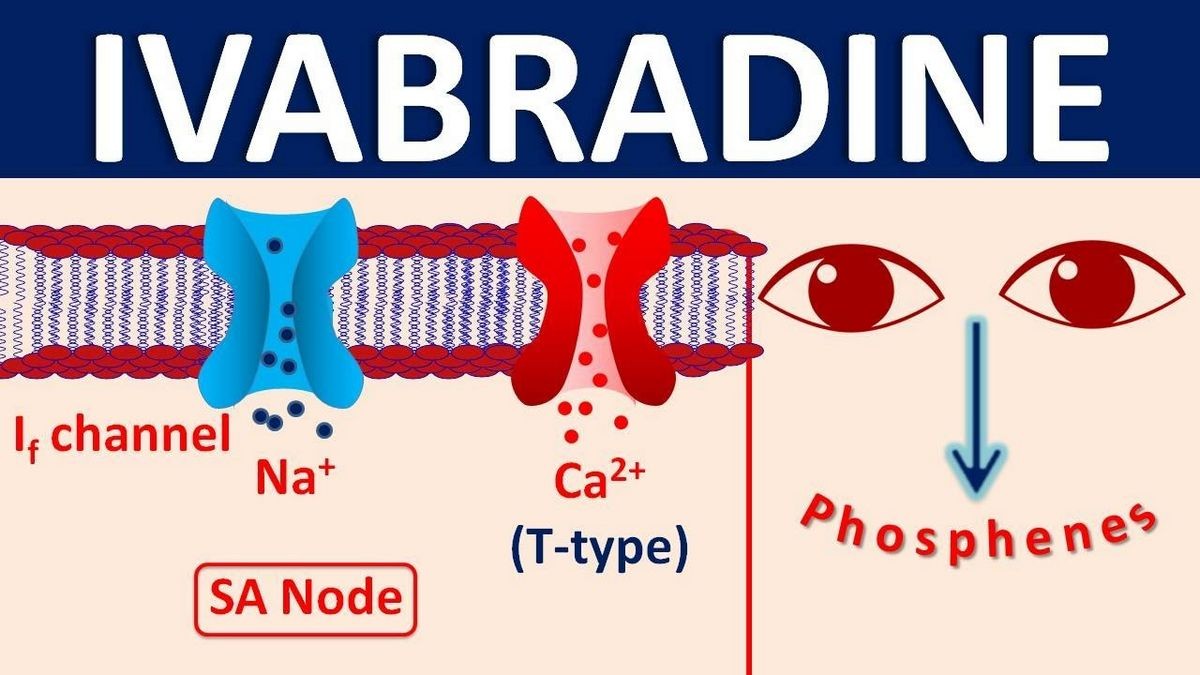
Corlanor is a hyperpolarization-activated cyclic nucleotide-gated channel blocker used to treat adults with chronic heart failure to reduce their risk of hospitalization, and to treat certain children with stable heart failure due to an enlarged heart.
Common side effects of Corlanor include increased blood pressure and temporary brightness in part of your field of vision.
Serious side effects of Corlanor include increased risk of irregular or rapid heartbeat (atrial fibrillation or heart rhythm problems) or slower than normal heart rate.
Drug interactions of Corlanor include grapefruit juice and St. John’s wort.
Corlanor is primarily metabolized by CYP3A4. Concomitant use of CYP3A4 inhibitors increases Corlanor plasma concentrations and use of CYP3A4 inducers decreases them.
Avoid concomitant use of CYP3A4 inducers such as St. John’s wort when using Corlanor.
The risk of slow heart rate increases with concomitant administration of drugs that slow heart rate. The use of Corlanor is not recommended in patients with demand pacemakers set to rates ≥ 60 beats per minute.
Corlanor may cause harm to a fetus. Females who are able to get pregnant must use effective birth control during treatment with Corlanor. Breastfeeding is not recommended while using Corlanor.
What are the important side effects of Corlanor (ivabradine)?
Corlanor may cause serious side effects in adults and children, including irregular or rapid heartbeat and slow heart rate.
In young children signs and symptoms of slow heart rate may include poor feeding, difficulty breathing or turning blue.
The most common side effects of Corlanor are increased blood pressure and temporary brightness in part of your field of vision, especially at night.
These are not all the side effects of Corlanor. Ask your doctor or pharmacist for more information. Call your doctor for medical advice about side effects.
Corlanor (ivabradine) side effects list for healthcare professionals
Clinically significant adverse reactions that appear in other sections of the labeling include atrial fibrillation, bradycardia, and conduction disturbances.
Clinical Trials Experience
In the SHIFT trial, safety was evaluated in patients treated with Corlanor and placebo.
The most common adverse drug reactions in the SHIFT trial are shown in Table 2.
Table 2: Adverse Drug Reactions with Rates ≥ 1.0% Higher on Ivabradine than Placebo occurring in > 1% on Ivabradine in SHIFT
| Ivabradine N = 3260 |
Placebo N = 3278 |
|
| Bradycardia | 10% | 2.2% |
| Hypertension, blood pressure increased | 8.9% | 7.8% |
| Atrial fibrillation | 8.3% | 6.6% |
| Phosphenes, visual brightness | 2.8% | 0.5% |
Luminous Phenomena (Phosphenes)
Phosphenes are transiently enhanced brightness in a limited area of the visual field, halos, image decomposition, colored bright lights, or multiple images.
Phosphenes are usually triggered by sudden variations in light intensity. Corlanor can cause phosphenes.
Onset is generally within the first 2 months of treatment, after which they may occur repeatedly.
Phosphenes were generally reported to be of mild to moderate intensity and led to treatment discontinuation in
Pediatric Patients With Heart Failure
The safety of Corlanor in pediatric patients 6 months to less than 18 years of age is based on a clinical trial in symptomatic heart failure patients with dilated cardiomyopathy and elevated heart rate.
This trial provides experience in patients treated with Corlanor for a median duration of 397 days, and patients given placebo. Bradycardia occurred at rates similar to those in adults. Phosphenes were observed in pediatric patients treated with Corlanor.
Postmarketing Experience
The following adverse reactions have been identified in adults during post-approval use of Corlanor: syncope, hypotension, torsade de pointes, ventricular fibrillation, ventricular tachycardia, angioedema, erythema, rash, pruritus, urticaria, vertigo, diplopia, and visual impairment.
What drugs interact with Corlanor (ivabradine)?
Cytochrome P450-Based Interactions
Corlanor is primarily metabolized by CYP3A4. Concomitant use of CYP3A4 inhibitors increases Corlanor plasma concentrations and use of CYP3A4 inducers decreases them.
The concomitant use of strong CYP3A4 inhibitors is contraindicated. Examples of strong CYP3A4 inhibitors include azole antifungals, macrolide antibiotics, HIV protease inhibitors, and nefazodone.
Avoid concomitant use of moderate CYP3A4 inhibitors when using Corlanor. Examples of moderate CYP3A4 inhibitors include diltiazem, verapamil, and grapefruit juice.
Avoid concomitant use of CYP3A4 inducers when using Corlanor. Examples of CYP3A4 inducers include St. John’s wort, rifampicin, barbiturates, and phenytoin.
Negative Chronotropes
The risk of bradycardia increases with concomitant administration of drugs that slow heart rate. Monitor heart rate in patients taking Corlanor with other negative chronotropes.
Pacemakers In Adults
Corlanor dosing is based on heart rate reduction, targeting a heart rate of 50 to 60 beats per minute in adults. The use of Corlanor is not recommended in patients with demand pacemakers set to rates ≥ 60 beats per minute.
Summary
Corlanor is a hyperpolarization-activated cyclic nucleotide-gated channel blocker used to treat adults with chronic heart failure and certain children with stable heart failure due to an enlarged heart. Common side effects of Corlanor include increased blood pressure and temporary brightness in part of your field of vision. Corlanor may cause harm to a fetus. Breastfeeding is not recommended while using Corlanor.