Contents
Zelapar (selegiline hydrochloride)
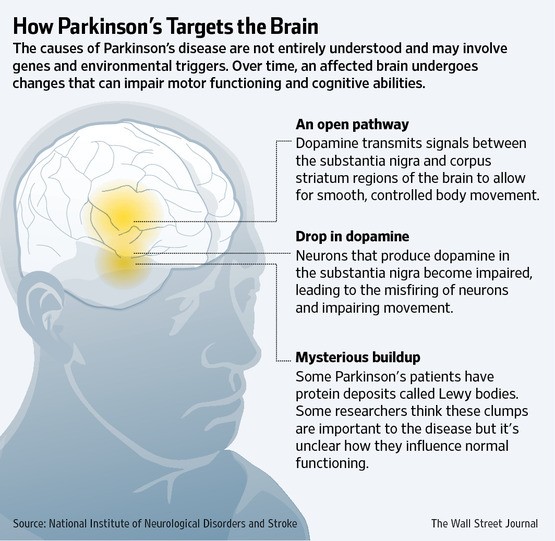
Zelapar (selegiline hydrochloride) is an enzyme blocker (MAO inhibitor) that slows the breakdown of neurotransmitters in the brain (such as dopamine, norepinephrine, and serotonin) used alongside other medicines to treat Parkinson’s disease symptoms.
Side effects of Zelapar
Common side effects include:
- dizziness
- abdominal pain
- dry mouth
- nausea
- stomach upset
- insomnia (trouble sleeping)
- headache
- weakness
- runny or stuffy nose
- back pain
- constipation
- redness/pain/swelling of the mouth/throat
- mouth sores or ulcers
- pain with swallowing
- fainting
- loss of balance
- mental/mood changes (agitation, confusion, depression, hallucinations)
- unusual strong urges (increased gambling, increased sexual urges)
- worsening muscle stiffness or twitching
- changes in sexual ability or interest
- increased shaking (tremor)
- swollen ankles or legs
- difficulty urinating
- unusual weight gain
- easy bleeding or bruising
- black or tarry stools
- vomit that looks like coffee grounds
Dosage for Zelapar
- Start with 1.25 mg once a day for at least 6 weeks. Increase to 2.5 mg once a day if desired benefit hasn’t been achieved and patient tolerates Zelapar.
- There’s no evidence that doses greater than 2.5 mg a day provide additional benefit and should typically be avoided due to potential increased risk of adverse events.
- Take Zelapar in the morning before breakfast without liquid. Avoid food or liquids for 5 minutes before and after taking Zelapar.
- Do not push Zelapar through the foil backing. Peel back one or two blisters with dry hands and gently remove the tablet(s). Immediately place the Zelapar tablet(s) on the tongue where it will disintegrate in seconds.
Patients With Hepatic Impairment
- In patients with mild to moderate hepatic disease (Child-Pugh score 5 to 9), reduce daily dose of Zelapar (from 2.5 to 1.25 mg) based on clinical response. Not recommended in patients with severe hepatic impairment (Child-Pugh score greater than 9).
Patients With Renal Impairment
- No dose adjustment of Zelapar is required in patients with mild to moderate renal impairment (creatinine clearance [CLcr] 30 to 89 mL/min). Maintenance dose of Zelapar (1.25 mg or 2.5 mg) is determined by individual clinical response.
- Not recommended in patients with severe renal impairment and end-stage renal disease (creatinine clearance [CLcr]
Drug interactions with Zelapar
Opioid Drugs
- Concomitant use of opioid drugs (e.g., meperidine, methadone, or tramadol) and MAO inhibitors, including selective MAO-B inhibitors like Zelapar, is contraindicated. Allow at least 14 days between discontinuation of Zelapar and initiation of treatment with these drugs.
Dextromethorphan
- Combining MAO inhibitors, including Zelapar, with dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior. Avoid concomitant use of dextromethorphan and Zelapar.
MAO Inhibitors
- Zelapar should not be used with other drugs in the MAOI class or other drugs that are potent inhibitors of monoamine oxidase because of increased risk for hypertensive crisis. Allow at least 14 days between discontinuation of Zelapar and initiation of treatment with other MAOIs.
Sympathomimetic Medications
- Taking the recommended dose of swallowed selegiline and a sympathomimetic medication (ephedrine) has been associated with uncontrolled hypertension, including hypertensive crisis.
Tyramine/Selegiline Interaction
- MAO inhibition in the gastrointestinal tract and liver can cause uncontrolled hypertension when ingesting tyramine-containing foods during Zelapar treatment. At recommended doses, Zelapar is relatively selective for MAO-B and does not typically require dietary tyramine restriction. Do not exceed 2.5 mg of Zelapar daily.
- Reports of hypertensive reactions have occurred with tyramine-containing consumables while on swallowed selegiline at the recommended dose. Hypertensive crisis has also been reported with Zelapar use within recommended dosing.
- Uncontrolled hypertension has been reported with the recommended dose of swallowed selegiline and a sympathomimetic medication (ephedrine).
Tricyclic Antidepressants And Selective Serotonin Reuptake Inhibitors
- Severe toxicity has been reported in patients taking tricyclic antidepressants or selective serotonin reuptake inhibitors with swallowed selegiline.
Drugs That Induce CYP450
- Use caution when using CYP3A4 inducers (e.g., phenytoin, carbamazepine, nafcillin, phenobarbital, and rifampin) as adequate studies haven’t been done to evaluate their effect on selegiline.
Dopaminergic Antagonists
- Dopamine antagonists, such as antipsychotics or metoclopramide, may reduce the effectiveness of Zelapar.
Is Zelapar safe during pregnancy or breastfeeding?
- No adequate data on developmental risk of Zelapar in pregnant women.
- In animal studies, selegiline administration during pregnancy was associated with developmental toxicity at doses higher than those used clinically.
- No data on presence of selegiline or its metabolites in human milk, effects on breastfed infant, or effects on milk production.
- Due to potential serious adverse reactions, including hypertensive reactions, breastfeeding is not recommended during Zelapar treatment and for 7 days after the final dose.
Summary
Zelapar (selegiline hydrochloride) is an enzyme blocker (MAO inhibitor) that slows the breakdown of neurotransmitters in the brain (such as dopamine, norepinephrine, and serotonin) used alongside other medicines to treat Parkinson’s disease symptoms. Common side effects include dizziness, abdominal pain, dry mouth, nausea, stomach upset, insomnia, headache, weakness, runny or stuffy nose, back pain, and constipation.